9 Lymphocyte Differentiation and Activation
9.1 Lymphocyte Development and Activation
9.1.1 Cytokines
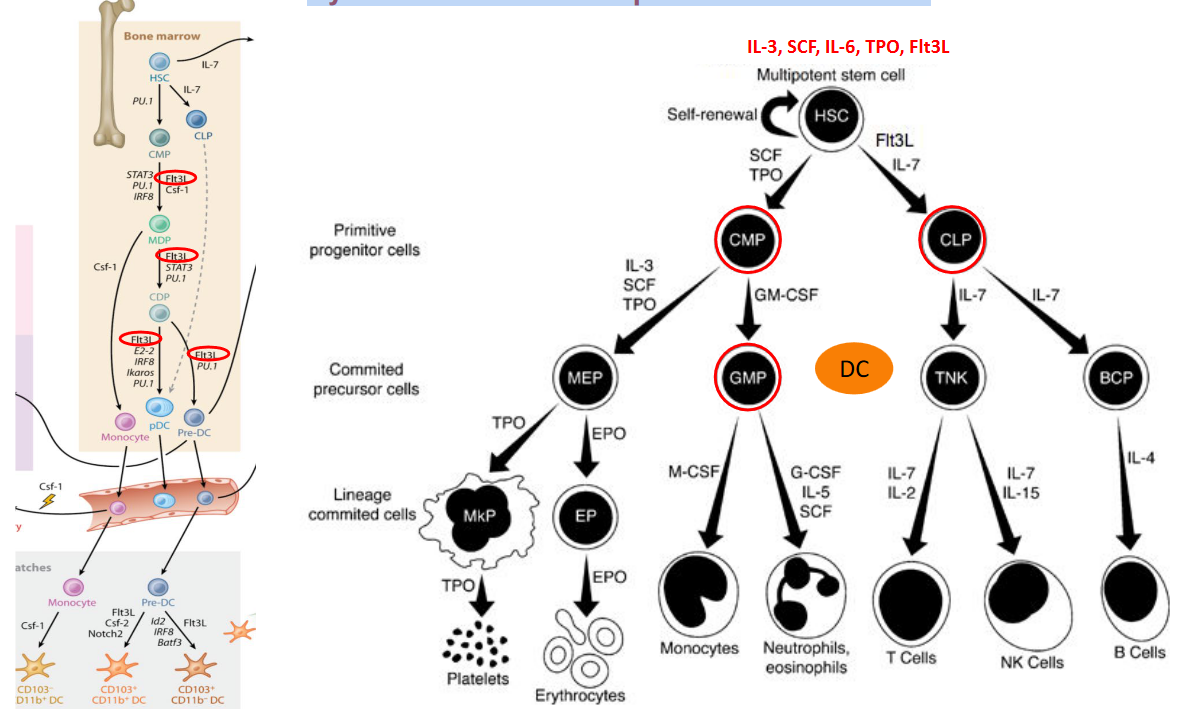
Cytokines play a crucial role in regulating hematopoietic differentiation, the process by which hematopoietic stem cells (HSCs) develop into various blood cell lineages. These signaling molecules are secreted by immune cells, stromal cells, and other cell types in the bone marrow microenvironment. By binding to specific receptors on hematopoietic progenitor cells, cytokines orchestrate a complex network of signaling pathways that control cell proliferation, differentiation, and survival.
One key function of cytokines in hematopoietic differentiation is their ability to direct lineage commitment, determining whether HSCs differentiate into myeloid, lymphoid, or erythroid lineages. For example, granulocyte-macrophage colony-stimulating factor (GM-CSF) promotes the differentiation of myeloid progenitor cells into granulocytes and macrophages, while interleukin-7 (IL-7) supports the development of lymphoid progenitor cells into B and T lymphocytes.
Furthermore, cytokines regulate the maturation and function of differentiated blood cells. For instance, erythropoietin (EPO) stimulates the proliferation and maturation of erythroid progenitor cells into red blood cells (erythrocytes), whereas interleukin-3 (IL-3) enhances the survival and function of various myeloid cells, including neutrophils, eosinophils, and basophils.
In addition to their role in lineage commitment and maturation, cytokines also regulate the balance between self-renewal and differentiation of HSCs. Certain cytokines, such as stem cell factor (SCF) and thrombopoietin (TPO), promote HSC self-renewal, ensuring the maintenance of a pool of multipotent stem cells capable of replenishing all blood cell lineages throughout life.
9.1.2 Epigenetic & Transcriptional Regulation
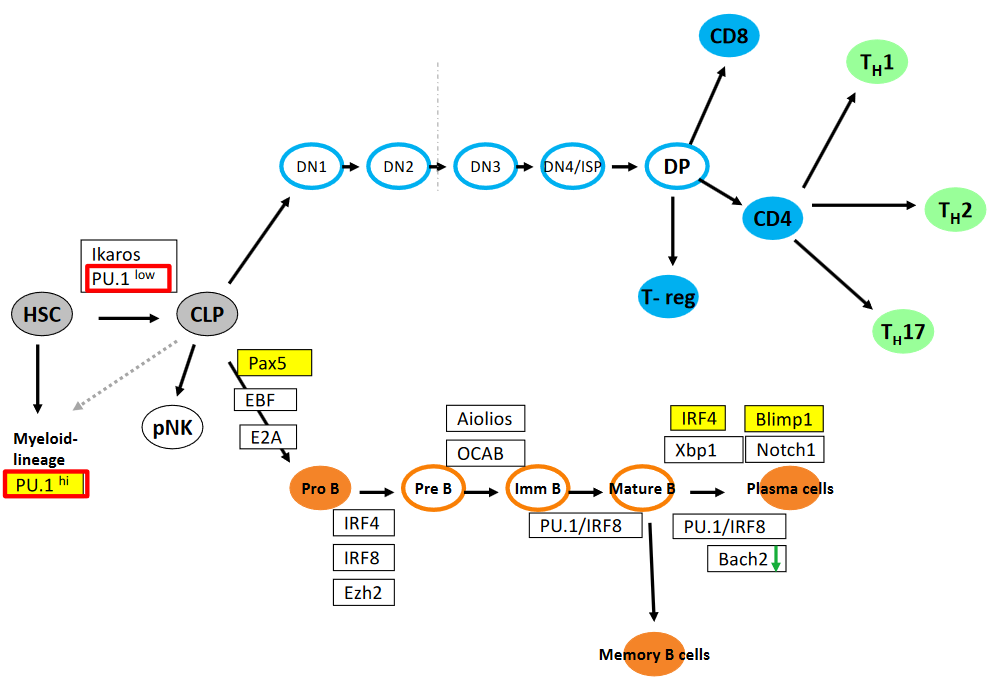
The transcription factors Ikaros, PU.1 (both low and high expression levels), Pax5, E2A, OCAB, Aiolios, IRF4, IRF8, Blimp1, Xbp1, Ezh2, and Bach2 play critical roles in the epigenetic and transcriptional regulation of hematopoiesis, particularly in the development and differentiation of various immune cell lineages.
Ikaros, PU.1 (in both low and high levels), Pax5, and E2A are involved in the regulation of early lymphoid progenitor cells, influencing lineage commitment and differentiation at distinct stages from DN1 to DN4/ISP. Additionally, IRF4, Notch1, and EBF contribute to the specification of different stages of lymphocyte development, particularly during the transition from double-negative (DN) to double-positive (DP) stages in thymocyte development.
In B cell differentiation, Pax5 and E2A play essential roles in commitment to the B cell lineage, while OCAB, Aiolios, and IRF4 are involved in the regulation of later stages of B cell development, including germinal center reactions and plasma cell differentiation. Blimp1, Xbp1, and Ezh2 are critical for plasma cell differentiation and antibody secretion, while Bach2 is involved in the maintenance of B cell homeostasis and prevention of terminal differentiation.
Moreover, the transcriptional regulators PU.1/IRF8 axis is crucial for myeloid cell development, including the specification of dendritic cells and macrophages. IRF4 and IRF8 also have roles in regulating the function of memory T cells and dendritic cells, respectively.
9.1.3 PU.1
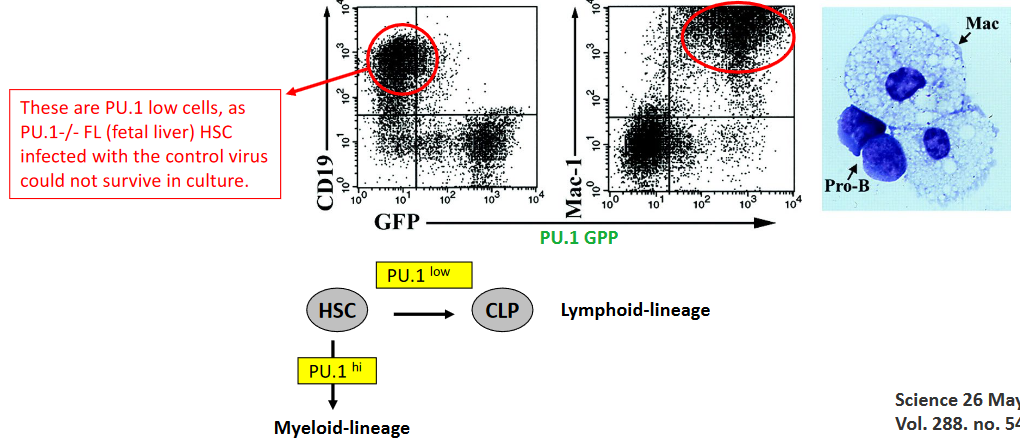
PU.1 is a critical transcription factor that plays a pivotal role in determining cell fate, particularly in the development of immune cell lineages. Complete knockout of PU.1 results in embryonic lethality at embryonic day 18 (E18) due to the absence of essential immune cell populations, including B cells, T cells, and macrophages. This underscores the indispensable role of PU.1 in the differentiation and development of these immune cell lineages.
In experiments involving PU.1-deficient fetal liver hematopoietic stem cells (HSCs), researchers have utilized a retrovirus expressing PU.1-GFP to introduce PU.1 into PU.1-deficient HSCs. These experiments were conducted using fetal liver HSCs infected with the PU.1-GFP retrovirus, with the infection carried out on stromal cells producing interleukin-7 (IL-7). IL-7-producing stromal cells provide a supportive environment for HSCs and facilitate their differentiation into various immune cell lineages. By infecting PU.1-deficient fetal liver HSCs with the PU.1-GFP retrovirus, researchers aim to restore PU.1 expression and investigate its impact on the differentiation potential and fate determination of these HSCs.
9.1.3.1 PU.1 Expression in Hematopoietic LIneage Cells
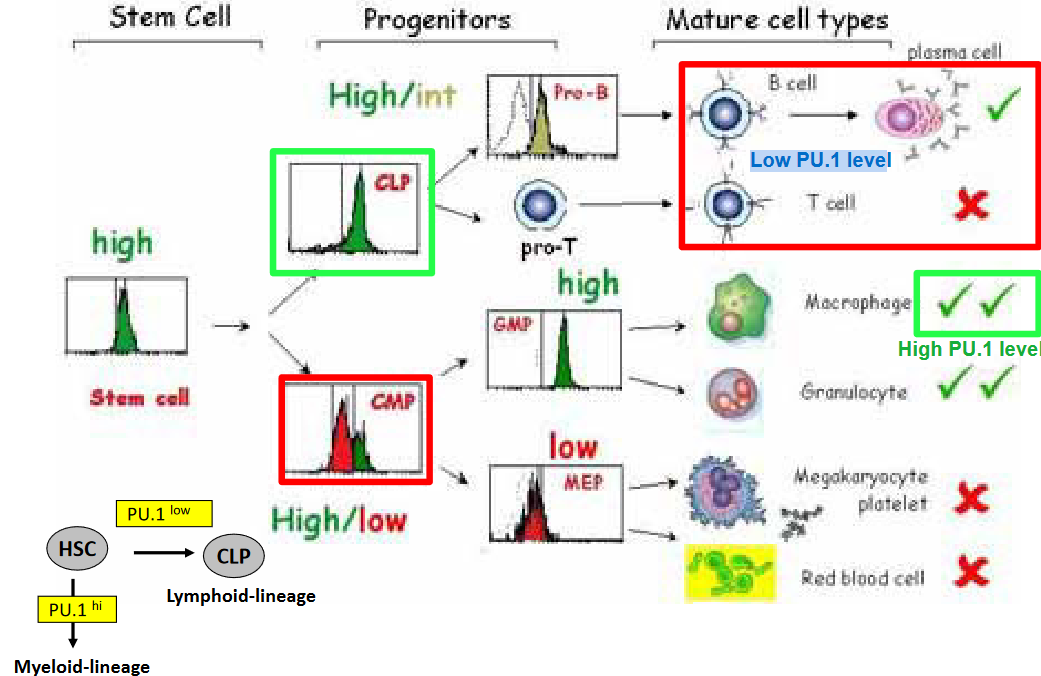
In hematopoietic stem cells (HSCs) and early progenitor cells, PU.1 expression is relatively low. This low PU.1 expression is associated with the maintenance of HSC self-renewal capacity and multipotency, allowing these cells to give rise to diverse blood cell lineages. Moreover, low levels of PU.1 are observed in early lymphoid progenitor cells, such as common lymphoid progenitors (CLPs), where PU.1 expression is downregulated to promote lymphoid lineage commitment and prevent myeloid differentiation.
Additionally, in mature lymphoid cells, such as B cells and T cells, PU.1 expression remains low or is silenced altogether. This low PU.1 expression is crucial for lineage-specific gene expression programs and functional specialization of lymphoid cells. For instance, in B cells, low PU.1 levels are necessary for the expression of B cell-specific transcription factors, such as Pax5 and EBF1, which orchestrate B cell development and function. Similarly, in T cells, low PU.1 expression is required for the expression of lineage-specific transcription factors, such as GATA3 and TCF-1, which regulate T cell differentiation and effector function.
9.1.4 Pax5
Pax5, a transcription factor critical for B cell development, plays a multifaceted role in orchestrating the commitment and differentiation of lymphoid progenitors into the B lymphocyte lineage. One of its key functions is to regulate the recombination of the distal V H gene segments within the immunoglobulin heavy chain (IgH) locus, a process essential for generating diverse antibody repertoires. Additionally, Pax5 is a crucial regulator of B cell identity, exerting control over various aspects of B cell development and function.
A notable role of Pax5 is its involvement in IgH locus contraction, a process that brings together the distal and proximal V H gene segments to facilitate V(D)J recombination. By facilitating this locus contraction, Pax5 contributes to the efficient rearrangement of immunoglobulin genes during B cell development, ensuring the generation of functional antibody diversity.
Furthermore, Pax5 acts as a master regulator of B cell fate determination by simultaneously repressing the expression of ‘inappropriate’ genes associated with other lineages and activating the expression of B lineage-specific genes. Through this dual role, Pax5 helps maintain the identity and function of mature B cells while preventing their differentiation into alternative cell lineages.
Studies have shown that Pax5-deficient pro-B cells exhibit impaired IgH locus contraction, leading to disruptions in V(D)J recombination and aberrant B cell development. However, when exogenous Pax5 expression is introduced into Pax5-deficient pro-B cells, it can restore IgH locus contraction to normal levels. This restoration of IgH locus contraction by exogenous Pax5 expression highlights the pivotal role of Pax5 in regulating the structural organization of the IgH locus during B cell development.
9.1.5 Blimp1 and Cell Differentiation

The requirement of Blimp1 (B lymphocyte-induced maturation protein 1) for plasma cell differentiation has been elucidated through various experimental approaches and observations in both in vitro and in vivo settings. Here are some key lines of evidence supporting the essential role of Blimp1 in plasma cell differentiation:
Gene knockout studies: Mice with targeted deletion of the Prdm1 gene, which encodes Blimp1, exhibit defects in plasma cell generation. These mice fail to develop mature plasma cells, leading to impaired antibody production and compromised humoral immune responses. This loss-of-function phenotype demonstrates the necessity of Blimp1 for plasma cell differentiation in vivo.
Overexpression studies: Conversely, overexpression of Blimp1 has been shown to promote plasma cell differentiation. Transgenic mice engineered to overexpress Blimp1 in B cells exhibit enhanced plasma cell formation and antibody secretion. This gain-of-function phenotype further underscores the pivotal role of Blimp1 in driving plasma cell differentiation.
Molecular mechanisms: Mechanistic studies have revealed the molecular pathways through which Blimp1 regulates plasma cell differentiation. Blimp1 acts as a transcriptional repressor that suppresses the expression of genes associated with B cell development and promotes the activation of genes involved in plasma cell maturation, including genes encoding immunoglobulins and plasma cell-associated factors.
Correlation with plasma cell markers: Blimp1 expression correlates temporally and spatially with the expression of plasma cell markers during B cell differentiation. Blimp1 is upregulated as B cells undergo terminal differentiation into plasma cells, and its expression is closely associated with the acquisition of plasma cell phenotype and function.
9.1.6 IRF4 and Blimp1

The role of IRF4 in plasma cell differentiation has been extensively studied, and it has been established that IRF4 plays a critical role in regulating this process. However, while IRF4 is known to regulate the expression of factors such as XBP-1, which is important for immunoglobulin transcription and high-rate antibody secretion, it does not directly control the expression of Blimp1.
Research published in Nature Immunology in 2006 has provided insights into the relationship between IRF4, Blimp-1 (B lymphocyte-induced maturation protein 1), and XBP-1 in plasma cell differentiation. This study demonstrated that IRF4 indeed regulates the expression of XBP-1, another transcription factor crucial for plasma cell differentiation. However, the study also found that XBP-1 alone is not solely responsible for plasma cell differentiation but is instead critical for facilitating immunoglobulin transcription, which is essential for high-rate antibody secretion.
Importantly, the study highlighted that while Blimp1 expression alone, without IRF4, is not sufficient to promote plasma cell differentiation, IRF4 is not directly involved in controlling Blimp1 expression. This indicates that while both IRF4 and Blimp1 are essential regulators of plasma cell differentiation, they operate through distinct molecular pathways and likely function independently of each other in this context.
9.1.7 Notch1 and its Importance
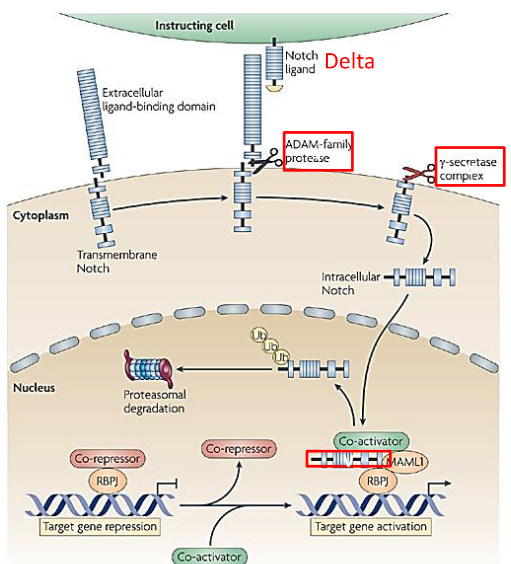
Notch1, a transmembrane receptor crucial for cell-cell communication, is indispensable for the development of T cells, particularly within the thymus, where T cell maturation primarily occurs. Through interactions with its ligands, such as Delta-like 4 expressed on thymic epithelial cells, Notch1 signaling initiates intracellular pathways that regulate T cell lineage commitment and differentiation. This signaling pathway is pivotal for driving multipotent progenitor cells towards the T cell lineage, inducing the expression of T cell-specific genes while suppressing alternative lineage programs.
In addition to its role in lineage commitment, Notch1 signaling influences the rearrangement of T cell receptor (TCR) genes during T cell development. Activation of Notch1 facilitates the recombination of TCR gene segments, contributing to the generation of diverse TCR repertoires crucial for antigen recognition and immune surveillance. Notably, Notch1 is particularly essential for αβ T cell development, the predominant subset of T cells involved in adaptive immune responses. Dysregulation or loss of Notch1 function leads to severe defects in αβ T cell development, resulting in impaired thymocyte maturation and reduced production of functional T cells.
9.1.7.1 Notch Deficient Cells
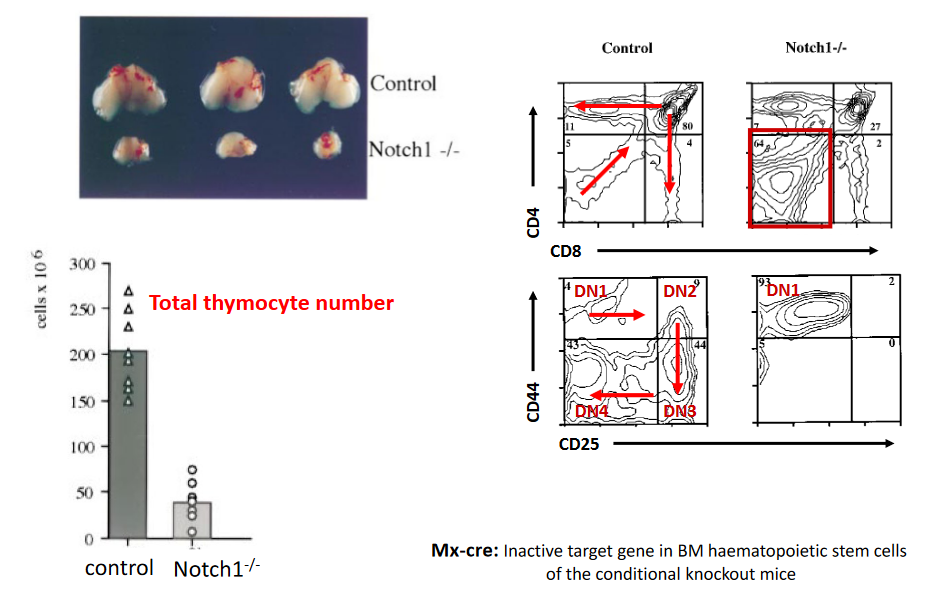
Mx-Cre-mediated deletion of Notch receptors in thymocytes leads to distinctive phenotypic alterations, offering insights into the crucial role of Notch signaling in T cell development. Notably, Notch-deficient thymocytes exhibit disruptions in various stages of T cell maturation within the thymus. One prominent phenotype observed in these thymocytes is a blockage or impairment in the transition from early T cell progenitors to more mature T cell populations. This blockade often manifests as a decrease or absence of CD4+CD8+ double-positive (DP) thymocytes, which are typically observed during the normal progression of T cell development.
Furthermore, the loss of Notch signaling in thymocytes can result in aberrant accumulation or expansion of specific T cell subsets, such as CD4-CD8- double-negative (DN) thymocytes or immature single-positive (ISP) thymocytes. These alterations in thymocyte populations suggest disruptions in the processes of lineage commitment and differentiation that are normally regulated by Notch signaling.
Additionally, Notch-deficient thymocytes may display defects in T cell receptor (TCR) gene rearrangement and expression, further contributing to their aberrant phenotype. Dysregulation of TCR gene rearrangement can impair the generation of functional TCR repertoires, compromising the ability of T cells to recognize and respond to antigens.
9.1.7.2 Determining Cell Fate
PU.1 is a master regulator of hematopoietic cell fate, essential for the development and differentiation of various immune cell lineages. Complete knockout of PU.1 results in profound defects in hematopoiesis, leading to embryonic lethality at embryonic day 18 (E18) in mice. These PU.1 knockout embryos exhibit a complete absence of critical immune cell populations, including B cells, T cells, and macrophages. This underscores the pivotal role of PU.1 in determining the fate of hematopoietic stem and progenitor cells and orchestrating the development of diverse immune cell lineages.
To investigate the role of PU.1 in hematopoiesis further, researchers have utilized fetal liver hematopoietic stem cells (HSCs) isolated from PU.1-deficient embryos. These PU.1-/- fetal liver HSCs were infected with a retrovirus expressing PU.1 fused with green fluorescent protein (GFP), allowing for the visualization of PU.1 expression. The retrovirus-mediated delivery of PU.1-GFP was carried out on stromal cells engineered to produce interleukin-7 (IL-7), a cytokine that supports the growth and differentiation of lymphoid progenitor cells.
By infecting PU.1-deficient fetal liver HSCs with the PU.1-GFP retrovirus, researchers aimed to restore PU.1 expression and investigate its impact on hematopoietic differentiation in vitro. This approach allowed for the examination of PU.1-mediated effects on lineage commitment and the generation of specific immune cell populations, such as B cells, T cells, and macrophages, under controlled experimental conditions.
9.1.7.3 T-Cell Differentiation and Notch1
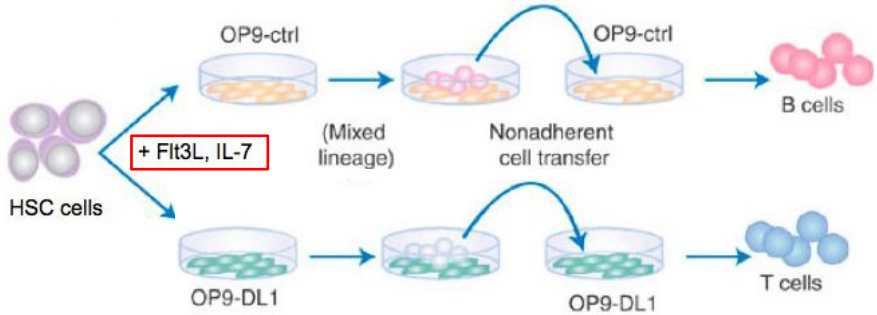
The ligand Delta-like 1 (Delta1) has been identified as an essential component for orchestrating T cell development within the thymus.
Studies utilizing genetic knockout or blocking approaches targeting Delta1 have provided compelling evidence for its indispensability in T cell differentiation. In the absence of Delta1 or upon blocking its interaction with Notch receptors, a significant impairment is observed in the transition of early thymic progenitors to more mature T cell populations. This blockade often results in a notable reduction or absence of CD4+CD8+ double-positive (DP) thymocytes, which are critical intermediates in the normal progression of T cell development.
Furthermore, the loss of Delta1-mediated Notch signaling disrupts the proper balance between T cell lineages, leading to aberrant accumulation or expansion of specific thymocyte subsets. This dysregulation suggests a pivotal role for Delta1-Notch signaling in guiding lineage commitment and differentiation processes during T cell development.
Moreover, Delta1-mediated Notch signaling is involved in regulating crucial events such as TCR gene rearrangement and expression, which are essential for generating functional T cell repertoires capable of recognizing and responding to antigens. Disruption of Delta1-Notch signaling can impair these processes, further compromising T cell development and function.
9.1.7.4 In Later Stages?
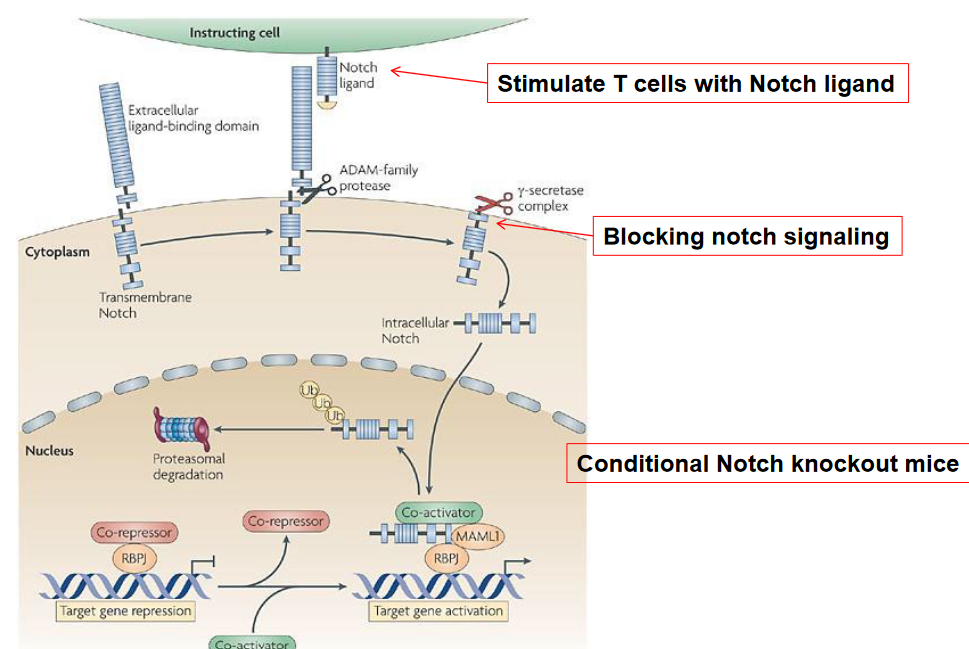
Once T cells have undergone maturation and emigrated from the thymus into the periphery, their dependence on Notch signaling diminishes compared to earlier developmental stages.
In the periphery, mature T cells encounter antigens presented by antigen-presenting cells (APCs) such as dendritic cells, macrophages, and B cells. Antigen recognition by the T cell receptor (TCR) triggers activation and subsequent effector functions, including cytokine secretion and cytotoxicity, depending on the T cell subset (CD4+ helper T cells or CD8+ cytotoxic T cells) and the nature of the antigen encountered.
While Notch signaling is generally less prominent in mature peripheral T cells, there are some contexts in which it may play a role. For example, Notch signaling has been implicated in certain effector functions of CD4+ T helper cells, such as Th1, Th2, and Th17 responses, as well as regulatory T cell (Treg) function. Notch signaling can influence the differentiation and cytokine production of these T cell subsets in response to specific environmental cues.
Moreover, in some pathological conditions, such as certain types of cancer or autoimmune diseases, dysregulated Notch signaling may contribute to aberrant T cell activation and function in the periphery. In these contexts, targeting Notch signaling pathways may have therapeutic implications.
9.1.7.5 Th1 Polarization
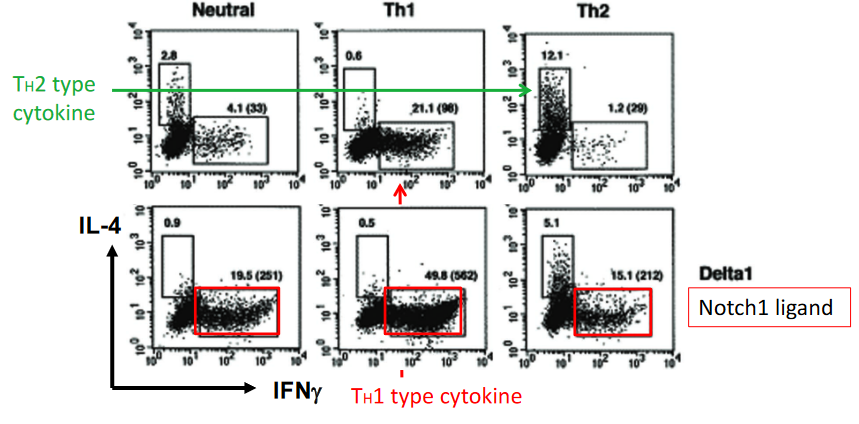
The effects of Delta, a Notch1 ligand, on T cell polarization highlight the intricate role of Notch signaling in regulating immune responses and T cell differentiation. Studies have shown that treatment with Delta, a ligand for Notch1, promotes Th1 polarization, characterized by the secretion of cytokines such as interferon-gamma (IFN-γ) and interleukin-2 (IL-2), which are associated with cellular immunity and inflammation.
Interestingly, while Notch signaling has been traditionally linked to Th2-type cytokine responses, such as interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-13 (IL-13), the observation that Delta treatment promotes Th1 polarization suggests a more nuanced role for Notch signaling in T cell differentiation. This indicates that Notch signaling can influence T cell polarization in a context-dependent manner, potentially modulating immune responses to different environmental cues or pathogenic challenges.
The precise mechanisms underlying the promotion of Th1 polarization by Delta treatment are not fully understood but may involve the modulation of transcriptional regulators or signaling pathways involved in T cell differentiation.
9.2 CD8+ and CD4+ Cells
9.2.1 CD8+ Cells
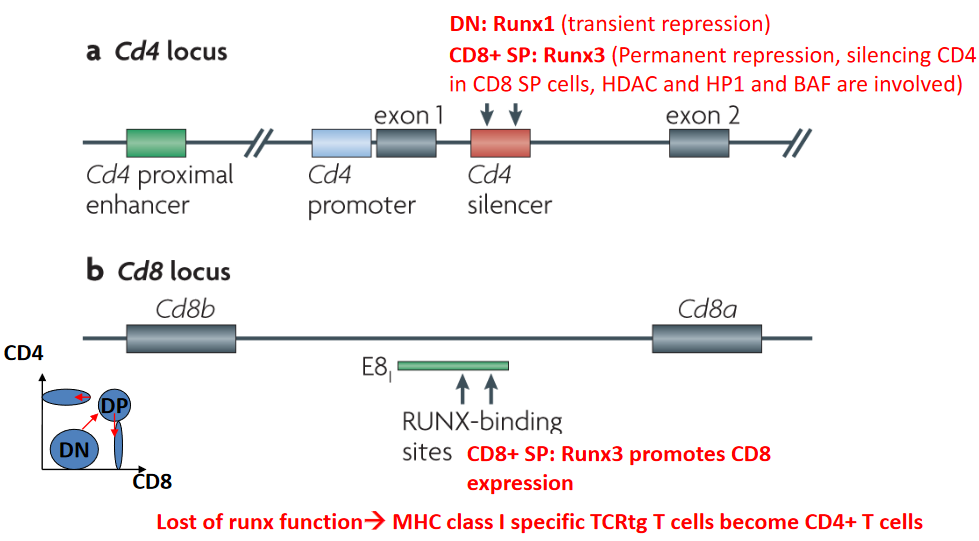
During T cell development, the differential expression and activities of transcription factors play critical roles in directing lineage commitment and cell fate decisions. In this context, the transcription factor Runx1 transiently represses the CD4 locus, contributing to the specification of CD8+ cytotoxic T cells. Runx-mediated repression of the CD4 locus is essential for the development of CD8+ single-positive (SP) T cells, which are specialized in recognizing antigenic peptides presented by major histocompatibility complex class I (MHC-I) molecules.
Following Runx1-mediated repression during the double-negative (DN) stage of T cell development, Runx3 assumes a crucial role in the permanent repression of the CD4 locus in CD8+ SP T cells. Unlike Runx1, Runx3’s repression of the CD4 locus is stable and enduring, ensuring the silencing of CD4 expression in mature CD8+ T cells. This process involves the recruitment of chromatin-modifying complexes, including histone deacetylases (HDACs), heterochromatin protein 1 (HP1), and the BRG1/BRM-associated factor (BAF) complex, which collectively contribute to the establishment and maintenance of a repressive chromatin state at the CD4 locus.
The functional importance of Runx transcription factors in directing T cell lineage commitment is underscored by studies demonstrating that loss of Runx function results in aberrant T cell development and differentiation.
9.2.2 CD4+ Cells
The transcription factor Th-POK (T-helper-inducing POZ/Krüppel-like factor) plays a crucial role in directing the differentiation of CD4+ T cells during T cell development. In the absence of Th-POK, CD4+ T cell development is impaired, leading to aberrant lineage commitment and differentiation. Conversely, Th-POK promotes the expression of CD4 and the development of mature CD4+ T cells by activating the CD4 locus and suppressing alternative lineage programs.
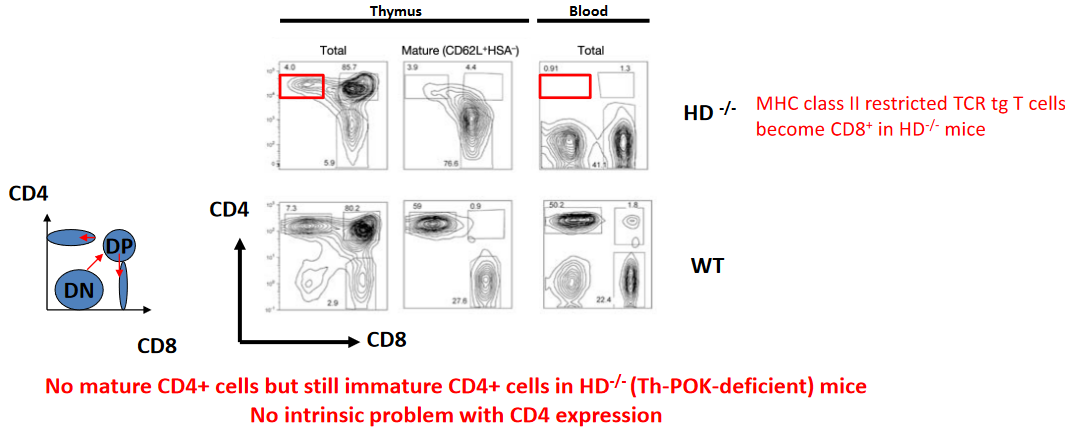
Studies using mouse models have revealed intriguing insights into the role of Th-POK in determining T cell fate. For instance, in mice with a deficiency in the major histocompatibility complex class II (MHC-II) molecule, which normally presents antigens to CD4+ T cells, T cells expressing MHC-II-restricted T cell receptor transgenes (TCRtg) unexpectedly differentiate into CD8+ T cells. This phenomenon suggests that the absence of MHC-II signaling can override the influence of Th-POK and promote CD8+ T cell differentiation, highlighting the intricate interplay between signaling pathways and transcriptional regulation in T cell development.
Moreover, studies using Th-POK-deficient mice have provided further insights into the role of Th-POK in CD4+ T cell development. In mice lacking Th-POK, mature CD4+ T cells are notably absent, indicating a crucial requirement for Th-POK in CD4 lineage commitment. However, intriguingly, immature CD4+ T cells are still present in these mice, suggesting that while Th-POK is essential for the maturation of CD4+ T cells, its absence does not intrinsically prevent CD4 expression.
9.2.2.1 Over-Expression of Th-POK
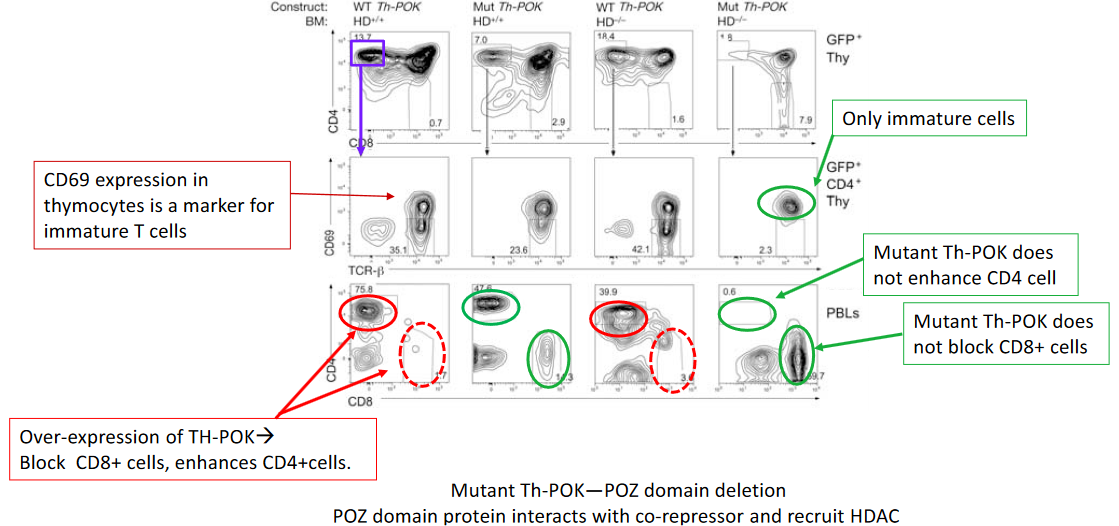
The intricate regulatory mechanisms governing CD4+ T cell differentiation involve the transcription factor Th-POK (T-helper-inducing POZ/Krüppel-like factor), which plays a pivotal role in orchestrating this process. Studies have demonstrated that overexpression of Th-POK enhances CD4+ T cell differentiation, underscoring its significance in directing lineage commitment and maturation.
One approach to elucidating the molecular mechanisms underlying Th-POK function involves the generation of mutant forms of Th-POK. For instance, deletion of the POZ (poxvirus and zinc finger) domain within Th-POK has been investigated to understand its role in CD4+ T cell differentiation. The POZ domain is known to facilitate interactions with corepressors, such as histone deacetylases (HDACs), thereby exerting transcriptional regulatory effects.
Studies utilizing mutant forms of Th-POK lacking the POZ domain have provided valuable insights into the mechanisms by which Th-POK regulates CD4+ T cell differentiation. By disrupting interactions with corepressors, such as HDACs, the mutant Th-POK may exhibit altered transcriptional activity, leading to aberrant gene expression patterns and potentially impacting CD4+ T cell differentiation.
9.2.3 Th1 and Th2 Differentiation

The differentiation of CD4+ T cells into distinct T helper (Th) cell subsets, such as Th1 and Th2 cells, is tightly regulated by changes in gene expression patterns, including modifications to cytokine loci. These modifications involve epigenetic mechanisms, such as DNA methylation and histone modifications, which play crucial roles in orchestrating the transcriptional programs that define each Th cell subset.
DNA methylation, the addition of a methyl group to cytosine residues within CpG dinucleotides, is one of the key epigenetic modifications associated with gene silencing. During Th cell differentiation, changes in DNA methylation patterns occur at cytokine loci, influencing the accessibility of these genes to transcription factors and RNA polymerase. For example, in Th1 and Th2 cells, DNA methylation changes can regulate the expression of key cytokines, such as IFN-γ and IL-4, respectively, thereby promoting the differentiation and function of each Th cell subset.
Similarly, histone modifications, including acetylation, methylation, phosphorylation, and ubiquitination, also play critical roles in regulating gene expression during Th cell differentiation. Histone modifications can alter chromatin structure and accessibility, thereby modulating the binding of transcription factors and the recruitment of transcriptional machinery to cytokine loci. For instance, histone acetylation, which is associated with active gene transcription, is often observed at cytokine loci in differentiated Th cells, facilitating the expression of cytokine genes characteristic of each Th cell subset.
9.2.4 T-bet

The transcription factor T-bet plays a crucial role in directing the differentiation of T helper 1 (Th1) cells, which are characterized by their production of interferon-gamma (IFN-γ) and their involvement in cell-mediated immunity. T-bet is essential for orchestrating the transcriptional program that drives Th1 cell differentiation and function.
Studies have shown that T-bet is specifically expressed in Th1 cells and is induced early during Th1 differentiation in response to signaling cues, such as interleukin-12 (IL-12) and interferon-gamma (IFN-γ). Once expressed, T-bet functions as a master regulator of Th1 cell differentiation by directly activating the expression of genes associated with the Th1 phenotype, including IFN-γ and other effector molecules.
Moreover, T-bet exerts its effects by promoting chromatin remodeling and enhancing the accessibility of Th1-specific gene loci, such as the Ifng gene encoding IFN-γ. Through its interactions with chromatin-modifying enzymes and transcriptional co-regulators, T-bet facilitates the assembly of transcriptional complexes that drive Th1-specific gene expression.
Additionally, T-bet regulates the expression of other transcription factors involved in Th1 differentiation, including signal transducer and activator of transcription 4 (STAT4) and interferon regulatory factor 1 (IRF1), further amplifying the Th1 differentiation program.
9.2.4.1 Without T-bet?
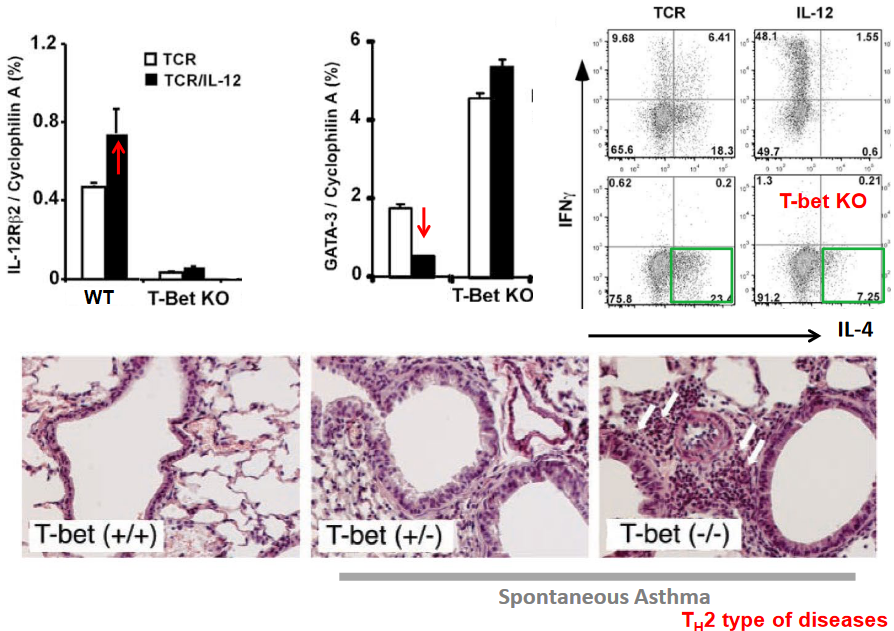
The absence of the transcription factor T-bet promotes the differentiation of T helper 2 (Th2) cells, which are characterized by their production of cytokines such as interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-13 (IL-13), and their involvement in allergic responses and immunity to helminth parasites. T-bet normally acts as a key regulator of Th1 cell differentiation, but its absence or downregulation allows for the preferential differentiation of Th2 cells.
Studies have shown that in the absence of T-bet, the balance of transcription factors shifts towards promoting Th2 cell differentiation. Without the inhibitory influence of T-bet, other transcription factors associated with Th2 cell differentiation, such as GATA3 and STAT6, are more readily expressed and activated. GATA3, in particular, is a master regulator of Th2 cell differentiation and is crucial for the expression of Th2-associated cytokines.
Furthermore, T-bet has been shown to directly repress the expression of genes associated with Th2 cell differentiation, including the gene encoding IL-4, a key cytokine driving Th2 responses. In the absence of T-bet, this repression is relieved, allowing for enhanced IL-4 production and subsequent Th2 cell differentiation.
9.2.5 ROR\(\gamma\)T

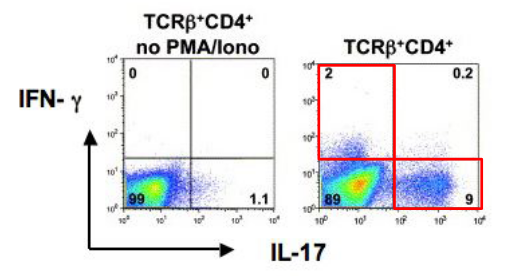
The transcription factor RORγt (retinoic acid receptor-related orphan receptor gamma t) plays a pivotal role in the differentiation of T helper 17 (Th17) cells, a subset of CD4+ T cells characterized by their production of interleukin-17 (IL-17) and involvement in immune responses against extracellular bacteria and fungi, as well as in autoimmune diseases. RORγt is considered a master regulator of Th17 cell differentiation and is critical for the expression of genes associated with the Th17 phenotype.
Upon activation by specific cytokine signals, such as transforming growth factor-beta (TGF-β) and interleukin-6 (IL-6), naïve CD4+ T cells upregulate RORγt expression, initiating the differentiation program towards the Th17 lineage. RORγt acts by binding to specific DNA sequences within the regulatory regions of target genes, thereby promoting their transcription and driving the expression of Th17-associated cytokines, including IL-17A, IL-17F, and IL-22.
Moreover, RORγt collaborates with other transcription factors, such as signal transducer and activator of transcription 3 (STAT3) and RORα, to further enhance the Th17 differentiation program. These transcriptional regulators form complex networks of interactions that fine-tune gene expression patterns and stabilize the Th17 cell phenotype.
The importance of RORγt in Th17 cell differentiation is underscored by studies demonstrating that mice lacking RORγt exhibit impaired Th17 cell development and are more susceptible to infections and autoimmune diseases characterized by dysregulated Th17 responses.
While T helper 1 (Th1) cells are essential for combating intracellular pathogens and regulating cell-mediated immune responses, their elimination alone does not fully account for the development of autoimmune diseases or the susceptibility to infections observed in animals. Other cell types within the immune system, including T helper 17 (Th17) cells, regulatory T (Treg) cells, and innate immune cells, also play critical roles in maintaining immune homeostasis and protecting against pathogens.
Th17 cells, characterized by their production of interleukin-17 (IL-17), are implicated in various autoimmune diseases, such as multiple sclerosis, rheumatoid arthritis, and inflammatory bowel disease. They contribute to tissue inflammation and damage by promoting the recruitment of immune cells to inflamed tissues and inducing the production of pro-inflammatory cytokines. Therefore, dysregulated Th17 responses can exacerbate autoimmune pathology.
Additionally, Treg cells are crucial for maintaining immune tolerance and preventing autoimmunity by suppressing excessive immune responses. Defects in Treg cell function or number can lead to loss of immune tolerance and the development of autoimmune diseases.
Furthermore, dysregulation of innate immune cells, such as macrophages and dendritic cells, can contribute to the pathogenesis of autoimmune diseases by promoting aberrant immune responses and tissue inflammation.
9.3 Genetically Modified Mice
Genetically modified mice are invaluable tools in biomedical research, providing insights into the functions of specific genes and their roles in health and disease. There are several approaches to generate genetically modified mice, including knockout/in mice, conditional knockout mice, and transgenic mice.
Knockout/in mice involve the targeted mutation of endogenous genes to disrupt their function (knockout) or introduce specific mutations (knock-in). Knockout mice lack the expression of a particular gene, allowing researchers to study the effects of gene loss on phenotype. Conversely, knock-in mice have a modified version of the gene inserted into their genome, mimicking genetic alterations found in human diseases or introducing reporter genes for visualization purposes.
Conditional knockout mice utilize genetic engineering techniques such as LoxP/Cre recombination to achieve spatial and temporal control over gene deletion. This approach allows researchers to target the mutation of endogenous genes selectively in specific cell types or at desired time points during development or adulthood. By using tissue-specific promoters to drive Cre recombinase expression or employing inducible Cre systems, researchers can study gene function in a cell- or stage-specific manner, overcoming potential embryonic lethality associated with global gene deletion.
Transgenic mice are generated by introducing foreign DNA, often containing an exogenous gene or regulatory elements, into the mouse genome. This results in the overexpression of the transgene in specific tissues or cell types. Transgenic mice are commonly used to investigate gene function, regulatory elements, and signaling pathways by assessing the effects of gene overexpression on phenotype.
9.3.1 Cre-LoxP System
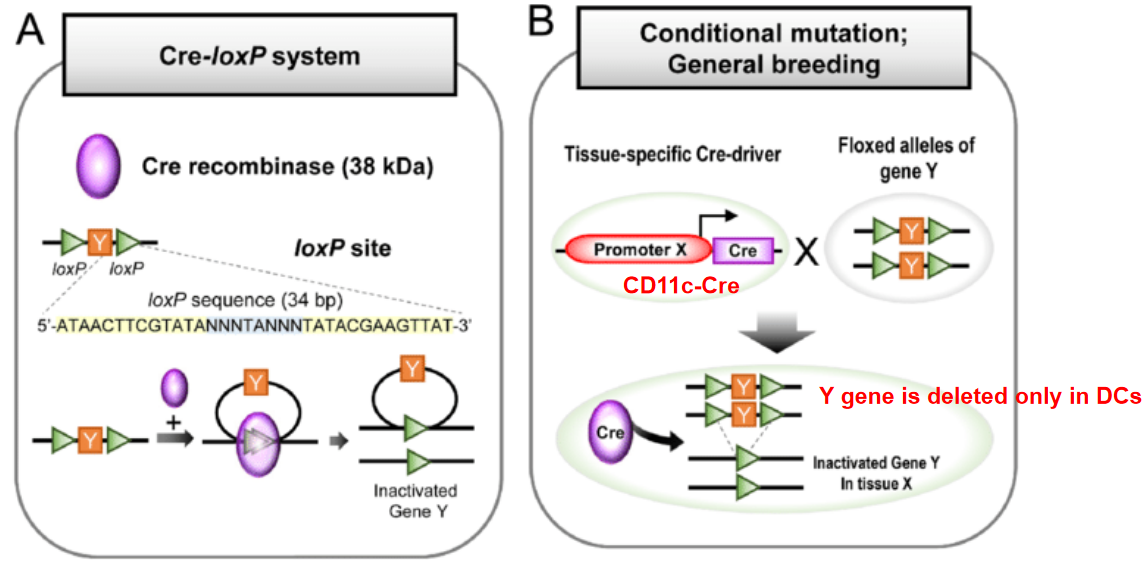
The Cre-LoxP system is a widely used genetic engineering tool that allows for the spatial and temporal control of gene expression or gene deletion in mice and other organisms. This system is based on the site-specific recombination activity of the Cre recombinase enzyme and its recognition of specific DNA sequences called LoxP sites.
The Cre recombinase enzyme recognizes and catalyzes recombination between pairs of short DNA sequences known as LoxP sites. These LoxP sites consist of palindromic sequences of 34 base pairs, each containing a central 8 base pair core region flanked by two 13 base pair inverted repeats. The core region is essential for Cre-mediated recombination.
In the Cre-LoxP system, two types of genetically modified mice are typically generated: mice carrying a gene of interest flanked by LoxP sites (floxed mice) and mice expressing Cre recombinase under the control of a tissue-specific or inducible promoter.
When Cre recombinase is expressed in cells containing floxed alleles, it catalyzes recombination between the LoxP sites, resulting in the excision of the DNA segment flanked by the LoxP sites. This process can lead to either the deletion or inversion of the intervening DNA sequence, depending on the orientation of the LoxP sites.
The Cre-LoxP system offers precise control over gene manipulation, allowing researchers to achieve tissue-specific or inducible gene deletion, activation, or inversion. By choosing appropriate promoters to drive Cre expression or employing inducible Cre systems, researchers can control the timing and location of gene modification, enabling the study of gene function in specific cell types or developmental stages.
9.4 Experimental Autoimmune Encephalomyelitis (i.e., EAE) and T-Reg Cells
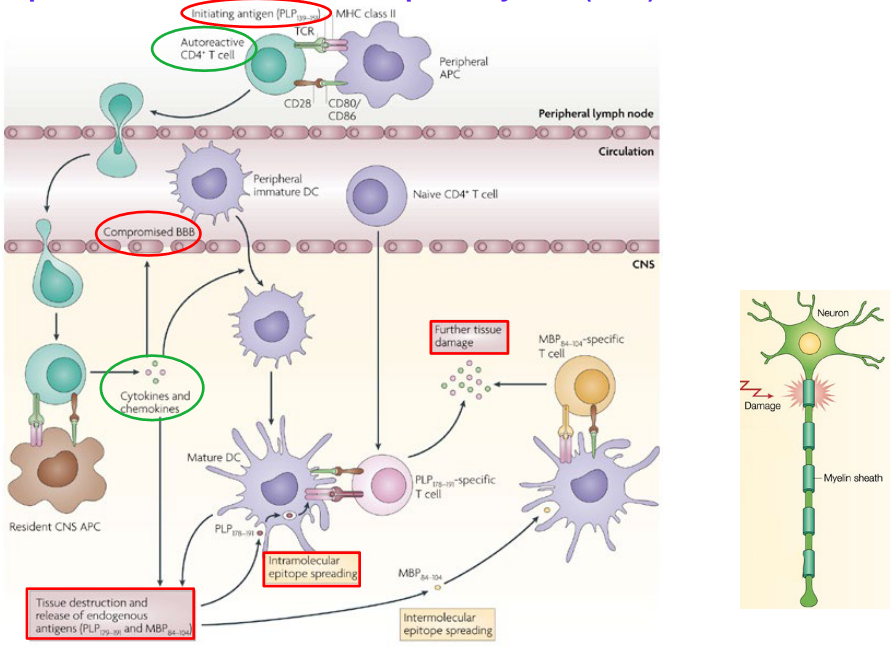
Experimental Autoimmune Encephalomyelitis (EAE) is a widely used animal model of multiple sclerosis (MS), a chronic autoimmune disease characterized by inflammation and demyelination in the central nervous system (CNS). EAE is induced in animals, typically mice or rats, by immunization with myelin antigens such as myelin basic protein (MBP), proteolipid protein (PLP), or myelin oligodendrocyte glycoprotein (MOG), combined with an adjuvant to enhance immune responses.
Following immunization, autoreactive T cells specific for myelin antigens are activated and infiltrate the CNS, where they recognize and attack myelin sheaths, leading to inflammation, demyelination, and neuronal damage. The clinical manifestations of EAE resemble those of MS and can include motor deficits, paralysis, and sensory disturbances.
EAE can be induced using various protocols, including active immunization with myelin antigens, passive transfer of autoreactive T cells or antibodies, or genetic manipulation to express myelin-specific T cell receptors. Different strains of mice or rats may exhibit variations in susceptibility and disease course, allowing researchers to study the genetic and environmental factors influencing disease susceptibility and progression.

Studies have demonstrated that mice deficient in the transcription factor RORγt (retinoic acid receptor-related orphan receptor gamma t), which is critical for the differentiation of T helper 17 (Th17) cells, are resistant to Experimental Autoimmune Encephalomyelitis (EAE), a widely used animal model of multiple sclerosis (MS). This finding suggests that Th17 cells play a key role in the pathogenesis and progression of EAE.
Th17 cells are known for their production of interleukin-17 (IL-17) and their involvement in promoting inflammation and tissue damage in autoimmune diseases, including MS. In the context of EAE, Th17 cells are thought to infiltrate the central nervous system (CNS) and contribute to the inflammatory response, leading to demyelination and neuronal damage characteristic of the disease.
The absence of RORγt in mice results in impaired differentiation and function of Th17 cells, leading to decreased production of IL-17 and reduced inflammatory responses in the CNS. Consequently, RORγt-deficient mice exhibit resistance to EAE induction compared to wild-type mice, highlighting the importance of Th17 cells in driving EAE disease progression.
9.4.1 Foxp3

Regulatory T (Treg) cells, characterized by the expression of the transcription factor Foxp3, play a crucial role in maintaining immune homeostasis and preventing autoimmune diseases by suppressing excessive or aberrant immune responses. Treg cells are central to the maintenance of immune tolerance, which is the ability of the immune system to recognize and tolerate self-antigens while mounting effective responses against foreign pathogens. Dysfunction or deficiency in Treg cells can lead to loss of immune tolerance and the development of autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, and type 1 diabetes.
Treg cells exert their suppressive function through various mechanisms, including the secretion of immunosuppressive cytokines such as interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β), direct cell-cell contact with effector T cells, and modulation of antigen-presenting cell function. By dampening immune responses, Treg cells help prevent excessive inflammation and tissue damage. Furthermore, Treg cells contribute to the maintenance of immune homeostasis by regulating the balance between effector T cells and regulatory T cells, crucial for the proper functioning of the immune system and the prevention of chronic inflammation and autoimmunity.
In addition to their role in preventing autoimmune diseases, Treg cells also play a role in suppressing anti-tumor immune responses, allowing tumors to evade immune surveillance and promote tumor growth and progression. In cancer immunotherapy, targeting Treg cells can enhance anti-tumor immune responses and improve the efficacy of immunotherapeutic interventions
9.4.1.1 Connections
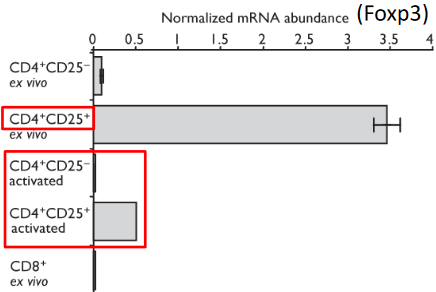

The transcription factor Foxp3 has garnered significant attention in the context of autoimmunity, both in mouse models and in humans. It is primarily associated with regulatory T (Treg) cells, a specialized subset of CD4+ T cells characterized by the expression of CD25. Foxp3 belongs to the forkhead/winged helix transcription factor family and is essential for the development and function of Treg cells.
Research has shown that Foxp3 is crucial for the generation of Treg cells, which play a pivotal role in maintaining immune tolerance and preventing autoimmune diseases. Dysfunction or deficiency in Foxp3 can lead to impaired Treg cell development and function, resulting in loss of immune tolerance and the development of autoimmunity. In mice and humans, mutations in the Foxp3 gene have been linked to severe autoimmune diseases such as immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, highlighting the critical role of Foxp3 in immune regulation and autoimmune pathogenesis.
The expression of Foxp3 in CD4+ CD25+ cells marks their commitment to the Treg cell lineage and is considered a hallmark of Treg cell identity.
9.4.1.2 Enlarged Lymphoid Organs
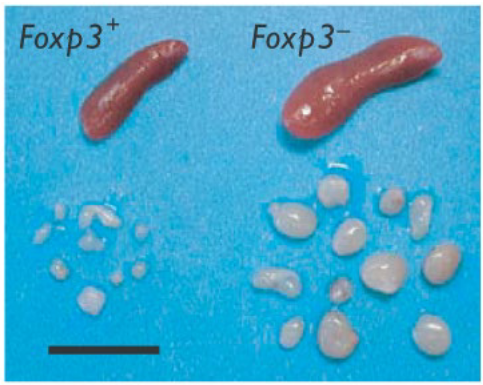
In the absence of Foxp3, significant alterations occur in the immune system, notably leading to the enlargement of lymphoid organs. Foxp3, a critical transcription factor for regulatory T (Treg) cell development and function, plays a pivotal role in maintaining immune tolerance and preventing autoimmune diseases. When Foxp3 is deficient or dysfunctional, the balance between regulatory T cells and effector T cells is disrupted, resulting in dysregulated immune responses.
Studies in mouse models and human patients with mutations in the Foxp3 gene have demonstrated that the absence of Foxp3 leads to the dysregulation of immune responses, characterized by unchecked activation of effector T cells and the breakdown of immune tolerance. This dysregulation manifests in various ways, including the enlargement of lymphoid organs such as the spleen and lymph nodes.
The enlarged lymphoid organs observed in the absence of Foxp3 reflect the dysregulated immune activation and proliferation of immune cells, including effector T cells, B cells, and antigen-presenting cells. This phenomenon is often accompanied by inflammation, tissue damage, and the development of autoimmune pathology in affected individuals.
9.4.2 Types of T-Reg Cells

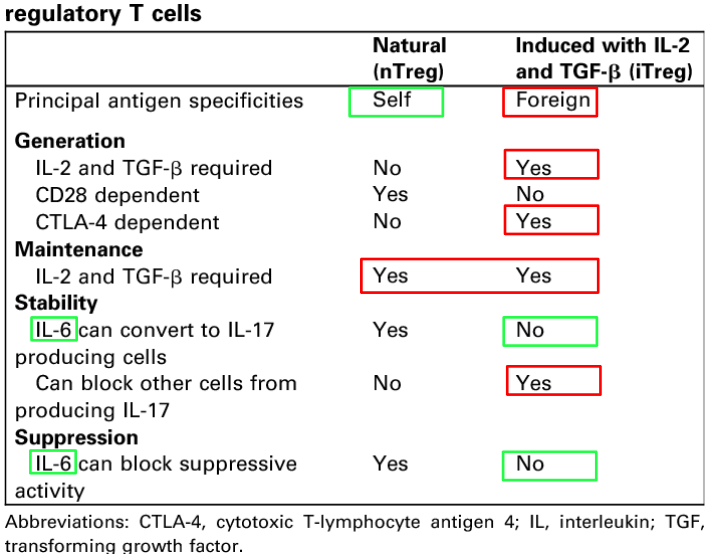
Among Treg cells, two main subsets are recognized based on their origin and development: natural regulatory T (nTreg) cells and induced regulatory T (iTreg) cells.
- Natural Regulatory T (nTreg) Cells:
- nTreg cells develop in the thymus during T cell maturation.
- They express high levels of the transcription factor Foxp3, which is essential for their development and suppressive function.
- nTreg cells recognize self-antigens and help maintain immune tolerance by suppressing autoreactive T cells that could otherwise attack healthy tissues.
- These cells constitute a fundamental component of the immune system’s intrinsic regulatory mechanisms.
- Induced Regulatory T (iTreg) Cells:
- iTreg cells differentiate from conventional CD4+ T cells (Tconv) in the periphery under specific conditions.
- Various factors, including cytokines such as transforming growth factor-beta (TGF-β) and interleukin-10 (IL-10), contribute to the induction of iTreg cells.
- Unlike nTreg cells, iTreg cells do not necessarily express Foxp3 constitutively but can acquire Foxp3 expression upon induction.
- iTreg cells are involved in maintaining immune tolerance at mucosal surfaces, where they help prevent excessive inflammation and immune-mediated tissue damage.
- The generation of iTreg cells can be influenced by environmental factors, such as the microbiota and inflammatory cytokines, highlighting their role in adaptive immune responses.
Both nTreg and iTreg cells contribute to immune homeostasis and tolerance, albeit through different developmental pathways and mechanisms.
9.4.3 Dendritic Cells Inducing Development of T-Reg Cells
Studies have shown that CD103+ DCs possess the unique ability to promote the generation of Foxp3+ Treg cells through a mechanism that relies on the actions of transforming growth factor-beta (TGF-β) and retinoic acid (RA). TGF-β is a potent immunosuppressive cytokine known for its ability to induce Treg cell differentiation, while RA, a metabolite of vitamin A, acts as a co-factor in this process.
The interaction between CD103+ DCs and naïve CD4+ T cells in mucosal tissues leads to the production of TGF-β and RA by the DCs. These immunoregulatory signals act synergistically to promote the expression of Foxp3, a master transcription factor for Treg cell development, in the responding T cells. As a result, the T cells differentiate into Foxp3+ Treg cells with suppressive functions.
The induction of Foxp3+ Treg cells by CD103+ DCs is an important mechanism for maintaining immune tolerance and preventing excessive inflammation and tissue damage in mucosal environments. This regulatory pathway helps to balance immune responses at mucosal surfaces, where interactions with commensal microorganisms and dietary antigens necessitate tight control to prevent inappropriate immune activation.
9.4.3.1 TGF-\(\beta\)
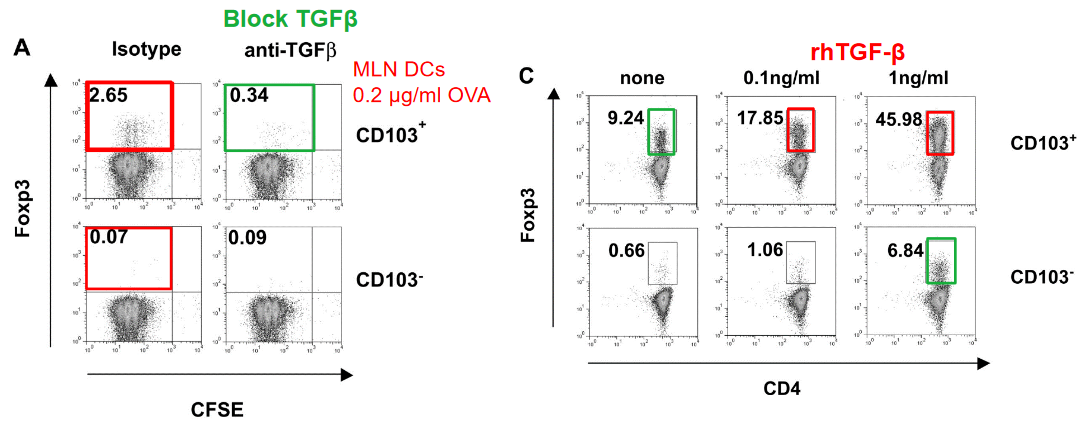
When naïve CD4+ T cells encounter TGF-β signaling in the presence of other environmental cues, such as inflammatory cytokines or co-stimulatory signals from antigen-presenting cells, they undergo a process of differentiation that leads to the upregulation of Foxp3 expression. This process is essential for the generation of functionally suppressive Treg cells capable of modulating immune responses and maintaining immune homeostasis.
TGF-β signaling acts by binding to its receptors on the surface of CD4+ T cells, initiating intracellular signaling cascades that culminate in the activation of specific transcriptional programs associated with Foxp3 expression. These signaling pathways drive the expression of Foxp3 and the acquisition of regulatory properties by the differentiating T cells, ultimately leading to their differentiation into mature Treg cells.
9.4.4 RA Cofactor

Retinoic acid (RA), a metabolite of vitamin A, serves as a critical cofactor in the induction of Foxp3, the master transcription factor of regulatory T (Treg) cells. Alongside transforming growth factor-beta (TGF-β), RA plays an essential role in promoting the differentiation of naïve CD4+ T cells into Foxp3-expressing Treg cells, particularly in mucosal tissues such as the intestine.
The presence of RA, together with TGF-β, creates an environment conducive to Treg cell generation, particularly within the gut-associated lymphoid tissue. RA functions by binding to retinoic acid receptors (RARs) expressed by CD4+ T cells, initiating signaling cascades that synergize with TGF-β signaling to promote Foxp3 expression and Treg cell differentiation.
RA contributes to the stabilization of the Foxp3 locus and enhances its transcriptional activity, thereby facilitating the establishment of a regulatory T cell phenotype. Importantly, RA also promotes the imprinting of gut-homing receptors on Treg cells, enabling them to migrate to and function within the intestinal mucosa, where they play a crucial role in maintaining immune tolerance and preventing excessive inflammation.