
8 Immunotherapy
Immunotherapy represents a revolutionary approach to treating diseases by harnessing the power of the body’s immune system. This innovative treatment strategy leverages various components of the immune system to combat diseases such as cancer, autoimmune disorders, infectious diseases, and graft-versus-host disease (GVHD).
In cancer immunotherapy, the immune system is activated or reprogrammed to recognize and target cancer cells specifically. Approaches may include immune checkpoint inhibitors, which release the brakes on immune cells, allowing them to recognize and attack cancer cells more effectively. Additionally, adoptive cell therapies, such as CAR-T cell therapy, involve modifying a patient’s own immune cells to recognize and destroy cancer cells.
Autoimmune diseases occur when the immune system mistakenly attacks healthy tissues and organs. Immunotherapy for autoimmune disorders aims to modulate or suppress the abnormal immune response to prevent damage to the body’s own tissues. This may involve immune-suppressing medications or biologics that target specific immune pathways involved in autoimmune diseases.
In the context of infectious diseases, immunotherapy can involve the administration of antibodies or vaccines to boost the body’s immune response against pathogens. Monoclonal antibodies, for example, can neutralize viruses or bacteria, while vaccines stimulate the immune system to produce protective antibodies against specific pathogens.
Graft-versus-host disease (GVHD) occurs when immune cells from a transplanted donor recognize the recipient’s tissues as foreign and mount an immune attack against them. Immunotherapy approaches for GVHD may involve immunosuppressive medications to dampen the donor immune response or regulatory T cell therapy to restore immune tolerance and prevent GVHD.
8.1 Cancer Immunosurveillance
Cancer immunosurveillance refers to the body’s natural ability to detect and eliminate cancerous cells through immune mechanisms. Studies in both mice and humans have provided evidence supporting the concept of cancer immunosurveillance.
In mice, experiments have shown that immunodeficient mice, which lack functional immune systems, exhibit an increased incidence of spontaneous tumors compared to wild-type mice with intact immune systems. Additionally, wild-type mice have been shown to be capable of rejecting chemically induced tumors, demonstrating the role of the immune system in recognizing and eliminating cancerous cells.
Similarly, in humans, immunodeficient individuals, such as those with congenital or acquired immunodeficiency disorders, have been found to have an increased risk of developing cancer. This increased cancer incidence in immunodeficient patients underscores the importance of the immune system in suppressing tumor development and progression.
Furthermore, aging is associated with a decline in immune function, a phenomenon known as immunosenescence. As individuals age, their immune systems may become less efficient at recognizing and eliminating cancerous cells, leading to an increased incidence of cancer with age. This age-related decline in immune function contributes to the higher cancer incidence observed in older individuals.
8.1.1 Spontaneous Tumor Development in WT Mice
Spontaneous tumor development is a phenomenon observed in both wild-type (normal, healthy) mice and immunodeficient mice. However, the frequency and types of tumors that arise can differ between these two groups.
In wild-type mice with intact immune systems, immunity plays a critical role in preventing cancer incidence. The immune system continually surveils the body for abnormal or transformed cells, detecting and eliminating them before they can develop into full-fledged tumors. This process, known as immunosurveillance, helps to maintain tissue homeostasis and prevent the emergence of cancer.
When the immune system is compromised, as in immunodeficient mice or individuals, the ability to detect and eliminate cancerous cells is impaired. As a result, these mice are more susceptible to developing tumors spontaneously. Without the surveillance and control mechanisms provided by the immune system, cancerous cells can proliferate unchecked, leading to the formation of tumors.
8.1.2 Anti-Tumor Effector Mechanisms
B cells, a component of the immune system traditionally associated with antibody production and humoral immunity, also play a role in anti-tumor effector mechanisms. Through the production of tumor-specific antibodies, B cells contribute to the recognition and elimination of cancerous cells. Two important mechanisms by which these antibodies exert anti-tumor effects are complement-mediated killing and antibody-dependent cellular cytotoxicity (ADCC).
In complement-mediated killing, tumor-specific antibodies bind to antigens present on the surface of cancer cells. This binding event triggers the activation of the complement cascade, a series of enzymatic reactions that culminate in the formation of the membrane attack complex (MAC). The MAC punctures the cell membrane of the targeted cancer cell, leading to cell lysis and death. This process is facilitated by the opsonization of the tumor cells by the antibodies, which enhances their recognition and destruction by immune cells.
In ADCC, tumor-specific antibodies bind to antigens on the surface of cancer cells, marking them for destruction. Immune cells such as natural killer (NK) cells possess Fc receptors that recognize the Fc portion of the bound antibodies. Upon binding to the Fc region of the antibody-coated tumor cells, NK cells release cytotoxic granules containing perforin and granzymes, which induce apoptosis in the cancer cells. This antibody-mediated activation of NK cells enhances their cytotoxic activity against the tumor cells, leading to their destruction.
8.1.3 Immune Cells
Anti-tumor effector mechanisms encompass a sophisticated interplay of immune cells and molecules aimed at recognizing and eliminating cancerous cells within the body. B cells, a key component of the adaptive immune system, contribute to this process by producing tumor-specific antibodies. These antibodies can initiate the destruction of cancer cells through complement-mediated killing, where the complement system is activated to lyse tumor cells, or through antibody-dependent cell-mediated cytotoxicity (ADCC), where immune cells such as natural killer (NK) cells are recruited to recognize and eliminate cancer cells targeted by antibodies.
Furthermore, the cellular arm of the immune system, including CD4+ T cells, CD8+ cytotoxic T lymphocytes (CTLs), and NK cells, plays a pivotal role in directly attacking cancer cells. CD8+ CTLs release cytotoxic molecules like perforin and granzymes, which induce apoptosis in cancer cells, while NK cells utilize similar mechanisms to induce cancer cell death. Additionally, NK cells can recognize and kill tumor cells through activating receptors like NKG2D. This direct cytotoxicity represents a crucial mechanism in the body’s defense against cancer.
Moreover, immune cells release cytokines such as interferon-gamma (IFNγ), granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor-alpha (TNFα), which orchestrate the anti-tumor response. These cytokines not only induce direct lysis of tumor cells but also inhibit angiogenesis, the formation of blood vessels that tumors depend on for growth, and recruit and activate other immune cells like dendritic cells, macrophages, and granulocytes to the tumor microenvironment, bolstering the immune response against cancer.
Dendritic cells, specialized antigen-presenting cells, are instrumental in initiating and coordinating anti-tumor immune responses. They capture antigens from cancer cells and present them to T cells, activating specific immune responses against the tumor. Furthermore, dendritic cells produce cytokines and chemokines that regulate the immune response and facilitate the recruitment of other immune cells to the tumor site.
8.2 Dendritic Cells
Dendritic cells (DCs), first characterized by Steinman and colleagues in 1972, are pivotal players in orchestrating immune responses within the body. Often referred to as the “pacemakers” of the immune system, DCs serve as crucial mediators in presenting antigens to both T and B lymphocytes, making them indispensable antigen-presenting cells (APCs). Their ability to capture, process, and present peptides and proteins derived from pathogens or other sources to immune cells initiates and shapes adaptive immune responses.
DCs exhibit diversity in their subtypes, each specialized for distinct functions within the immune system. These subtypes include various populations found in different tissues and organs, each with unique phenotypic and functional characteristics. The classification of DCs encompasses subsets such as conventional dendritic cells (cDCs), plasmacytoid dendritic cells (pDCs), and migratory DCs, each playing specialized roles in immune surveillance and activation.
DCs exist in different states of maturation, ranging from immature to mature phenotypes. Immature DCs are adept at capturing antigens from their surroundings but exhibit limited capacity for antigen presentation and T cell activation. Upon encountering danger signals, such as pathogen-associated molecular patterns (PAMPs) or inflammatory cytokines, immature DCs undergo maturation, upregulating co-stimulatory molecules and enhancing antigen-presenting capacity to efficiently prime T cells and initiate immune responses.
Importantly, DCs can exhibit distinct functional states, termed immunogenic and tolerogenic DCs, depending on the microenvironment and stimuli encountered. Immunogenic DCs promote immune responses by activating T cells and inducing effector functions, while tolerogenic DCs play a critical role in maintaining immune tolerance and preventing excessive immune activation, thereby preventing autoimmune responses and promoting immune homeostasis.
8.2.1 In Cancer Immunotherapy?
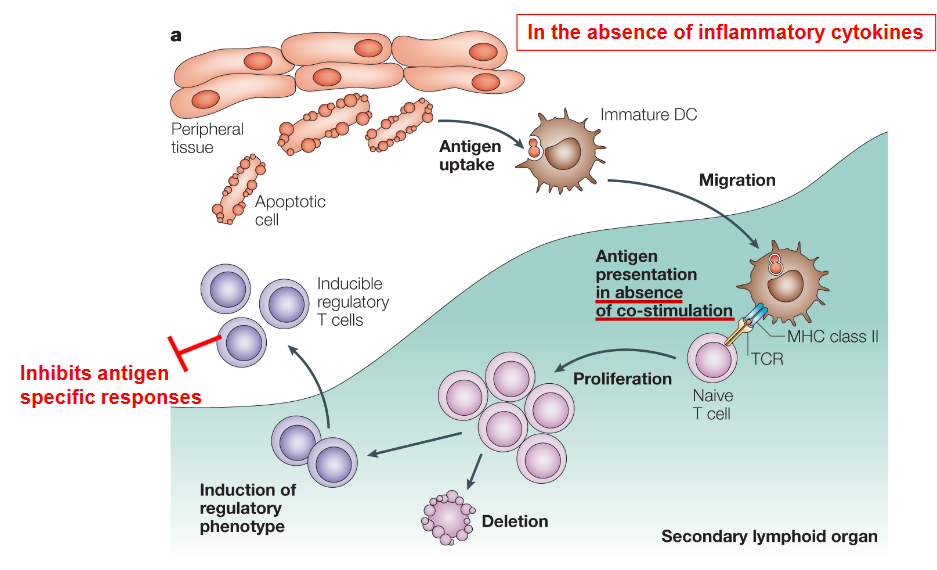
Immunogenic DCs are adept at promoting immune responses by activating T and B cells, making them valuable targets in cancer immunotherapy. These DCs efficiently present tumor antigens to T cells, initiating potent anti-tumor immune responses. Strategies aimed at enhancing the immunogenicity of DCs, such as DC-based vaccines or immune checkpoint blockade therapies, leverage the ability of immunogenic DCs to stimulate robust anti-tumor immune responses, offering promising approaches for cancer treatment.
Conversely, tolerogenic DCs, such as CD103+ DCs found in the lamina propria of the intestine, are essential for maintaining immune tolerance and preventing excessive immune activation. These DCs play a critical role in preventing autoimmune responses by inducing regulatory T cell (Treg) expansion and suppressing effector T cell responses. In the context of infectious diseases, tolerogenic DCs may contribute to immune evasion by pathogens, highlighting their role in pathogen-host interactions.
Understanding the interplay between immunogenic and tolerogenic DCs is crucial for the development of immunotherapy strategies tailored to specific diseases. In cancer immunotherapy, targeting immunogenic DCs can enhance anti-tumor immune responses, while in autoimmune diseases, promoting tolerogenic DC function may help dampen aberrant immune responses and restore immune tolerance. Additionally, strategies aimed at inducing transplant tolerance rely on tolerogenic DCs to prevent graft rejection and promote long-term graft survival.
8.2.1.1 In Presence of Inflammatory Cytokine?

In the presence of inflammatory cytokines, dendritic cells (DCs) undergo a process known as maturation, which enhances their ability to activate and orchestrate immune responses. Inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and interferons (IFNs), are often produced in response to infection, tissue damage, or other immune stimuli, and they serve as signals to alert the immune system to potential threats.
When exposed to inflammatory cytokines, immature DCs undergo phenotypic and functional changes that promote their maturation into fully activated antigen-presenting cells. These changes include upregulation of co-stimulatory molecules such as CD80, CD86, and major histocompatibility complex (MHC) molecules, as well as increased production of pro-inflammatory cytokines and chemokines.
Maturation of DCs in response to inflammatory cytokines enhances their antigen-presenting capacity, allowing them to efficiently capture, process, and present antigens to T cells. Mature DCs migrate to secondary lymphoid organs, such as lymph nodes, where they encounter and activate T cells. The upregulation of co-stimulatory molecules on mature DCs provides the necessary signals to T cells for their activation and proliferation.
In addition to activating T cells, mature DCs also produce cytokines and chemokines that contribute to the inflammatory response and help recruit other immune cells to the site of infection or tissue damage. This amplifies the immune response and promotes the clearance of pathogens or damaged cells.
Furthermore, mature DCs play a crucial role in shaping the differentiation of T cells into various effector subsets, such as Th1, Th2, or regulatory T cells (Tregs), depending on the cytokine milieu present during T cell activation. This process allows for the generation of tailored immune responses tailored to the specific type of pathogen or immune challenge encountered.
8.2.1.2 Rationale

One key rationale for DC immunotherapy involves ex vivo culture techniques aimed at enhancing DC function and antigen presentation capabilities.
In cancer patients, dendritic cells may fail to adequately recognize tumor antigens or induce robust T cell responses due to immune evasion mechanisms employed by tumors or the suppressive tumor microenvironment. Ex vivo culture techniques allow for the isolation and manipulation of DCs outside the body, providing an opportunity to enhance their function and antigen-presenting capacity.
During ex vivo culture, DCs can be loaded with tumor-specific antigens, either derived from the patient’s own tumor cells or synthetic peptides representing tumor-associated antigens. By exposing DCs to tumor antigens in vitro, they are primed to present these antigens to T cells in a manner that activates and expands tumor-specific T lymphocytes.
After loading with tumor antigens, the cultured DCs are then transferred back to the patient, where they serve as potent stimulators of the immune response against the tumor. These “educated” DCs can effectively initiate and expand tumor-responsive T lymphocytes, including cytotoxic CD8+ T cells capable of directly killing tumor cells and helper CD4+ T cells that support anti-tumor immune responses.
8.2.2 Improvements
To enhance the cytotoxic T cell response, strategies should focus on optimizing antigen presentation by DCs to prime and activate cytotoxic CD8+ T cells more effectively. This could involve selecting potent tumor antigens or epitopes, optimizing antigen loading techniques, and incorporating adjuvants or co-stimulatory molecules to enhance T cell activation.
Memory T cell protection is crucial for long-lasting immune responses against tumors. Improvements in DC immunotherapy protocols should aim to induce durable memory T cell responses by promoting the generation and maintenance of memory T cell populations. This may involve selecting antigenic targets that elicit robust memory T cell responses and optimizing vaccination schedules to promote memory T cell formation.
Unwanted regulatory T cell (Treg) function can dampen anti-tumor immune responses and hinder the effectiveness of DC immunotherapy. Strategies to mitigate Treg-mediated suppression may include the use of immunomodulatory agents or targeting Treg-specific pathways to inhibit their suppressive activity selectively.
Addressing the laborious and expensive nature of ex vivo culturing is essential for the widespread adoption of DC immunotherapy. In vivo antigen loading of DCs represents a promising alternative approach that could simplify and expedite the production of therapeutic DCs. This approach involves directly targeting DCs in vivo with tumor antigens or utilizing methods such as nanoparticle-based delivery systems to deliver antigens to DCs within the body.
8.2.3 Antigen Loading Mechanism

The process of in vivo antigen loading of DCs typically begins with the administration of tumor antigens or antigenic components, such as peptides or proteins, into the body through various routes, such as injection, oral administration, or topical application. These antigens may be derived from the patient’s own tumor cells, tumor lysates, recombinant proteins, or synthetic peptides representing tumor-specific epitopes.
Once introduced into the body, the tumor antigens encounter DCs present in peripheral tissues, lymphoid organs, or circulation. In response to the presence of antigens and associated danger signals, such as pathogen-associated molecular patterns (PAMPs) or inflammatory cytokines, DCs undergo a process of maturation and activation.
Maturation signals trigger changes in DC phenotype and function, including upregulation of co-stimulatory molecules (e.g., CD80, CD86) and major histocompatibility complex (MHC) molecules, as well as increased production of pro-inflammatory cytokines and chemokines. These changes enhance the antigen-presenting capacity of DCs and promote their migration to secondary lymphoid organs, such as lymph nodes, where they interact with T cells.
During this process, mature DCs capture and process tumor antigens, presenting them to T cells in the context of MHC molecules. This interaction leads to the activation and expansion of tumor-specific T cells, including cytotoxic CD8+ T cells capable of directly killing tumor cells and helper CD4+ T cells that support anti-tumor immune responses.
8.2.4 Cancer Vaccines
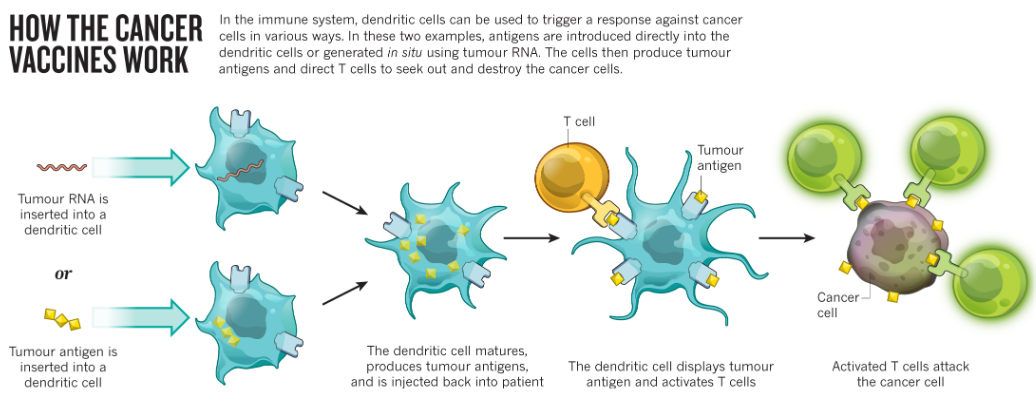
Establishing a cell line from the patient’s tumor is a crucial step in understanding the tumor’s characteristics and testing its susceptibility to various drugs. By culturing tumor cells in the laboratory, researchers can assess how different drugs affect tumor growth and viability, aiding in the selection of the most effective treatment strategies tailored to the patient’s tumor biology. This approach provides valuable insights into potential therapeutic options and helps guide personalized treatment decisions.
Sequencing the tumor’s DNA provides valuable information about the genetic mutations or alterations present in the tumor. By analyzing the tumor genome, researchers can identify specific genetic changes that may drive tumor growth or confer resistance to certain drugs. This personalized approach to treatment allows for the selection of targeted therapies that are most likely to be effective based on the genetic characteristics of the tumor, potentially improving treatment outcomes and minimizing adverse effects.
8.3 Tumor Antigens
Tumor antigens play a critical role in stimulating immune responses against cancer cells. There are two main categories of tumor antigens: tumor-specific antigens (TSAs) and tumor-associated antigens (TAAs). TSAs are unique to cancer cells and are not found in normal cells, making them attractive targets for immunotherapy. TAAs, on the other hand, are proteins that are overexpressed or aberrantly expressed in cancer cells compared to normal tissues.
However, identifying suitable tumor antigens for immunotherapy presents several challenges. One challenge is identifying tumor antigens or peptides that can be recognized by T cells. These antigens may arise from mutated proteins, fusion proteins resulting from chromosomal translocations, or abnormal expression of normal proteins. Another challenge is that specific tumor antigens or peptides often only work for particular human leukocyte antigen (HLA) types, also known as major histocompatibility complex (MHC) types. HLA molecules present antigens to T cells, and the compatibility between tumor antigens and HLA types determines whether T cells can recognize and respond to the tumor.
Overcoming these challenges requires comprehensive strategies for antigen discovery and characterization. Advanced techniques, such as next-generation sequencing and mass spectrometry, can be employed to identify mutated or aberrantly expressed proteins in tumor cells. Additionally, bioinformatics tools can help predict potential tumor antigens based on genomic and proteomic data. Extracting peptides from the tumor’s surface enables the identification of tumor-specific antigens that can be used to stimulate the patient’s immune system. These tumor-derived peptides serve as targets for vaccination, aiming to activate the immune system to recognize and destroy tumor cells. Vaccination with tumor antigens helps initiate an anti-tumor immune response, potentially enhancing the body’s ability to fight the disease and improving overall treatment efficacy.
8.4 Tumor Microenvironment

8.4.1 Tumor Promoting Factors
Chronic inflammation within the tumor microenvironment leads to the secretion of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 (IL-1). These cytokines promote tumor growth and progression by stimulating cell proliferation, angiogenesis, and metastasis.
Tumor-infiltrating immune cells, including tumor-associated macrophages (TAM) and myeloid-derived suppressor cells (MDSC), exhibit an immunosuppressive phenotype. They suppress anti-tumor immune responses through various mechanisms, including the secretion of immunosuppressive cytokines like transforming growth factor-beta (TGF-β) and interleukin-35 (IL-35), as well as contact-dependent mechanisms.
T cells within the tumor microenvironment often express inhibitory receptors such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1), which dampen T cell activation and effector functions, contributing to immune evasion by the tumor.
Regulatory T cells (Tregs) are another immune cell population present in tumors that suppress immune responses, further aiding tumor immune evasion. Tregs secrete immunosuppressive cytokines like TGF-β and IL-10 and exert their suppressive effects through contact-dependent mechanisms.
Tumor cells themselves contribute to the immunosuppressive microenvironment by secreting immunosuppressive cytokines such as IL-10 and TGF-β, as well as vascular endothelial growth factor (VEGF), which promotes angiogenesis and tumor vascularization. Tumor cells may also express programmed death-ligand 1 (PD-L1), which interacts with PD-1 on T cells to inhibit their function and promote immune evasion.
Participating in experimental treatments based on dendritic cells (DCs) offers the patient the opportunity to explore novel therapeutic approaches that harness the immune system to target the tumor. DC-based immunotherapy aims to activate anti-tumor immune responses by loading DCs with tumor antigens and reintroducing them into the patient.
8.4.2 PD1
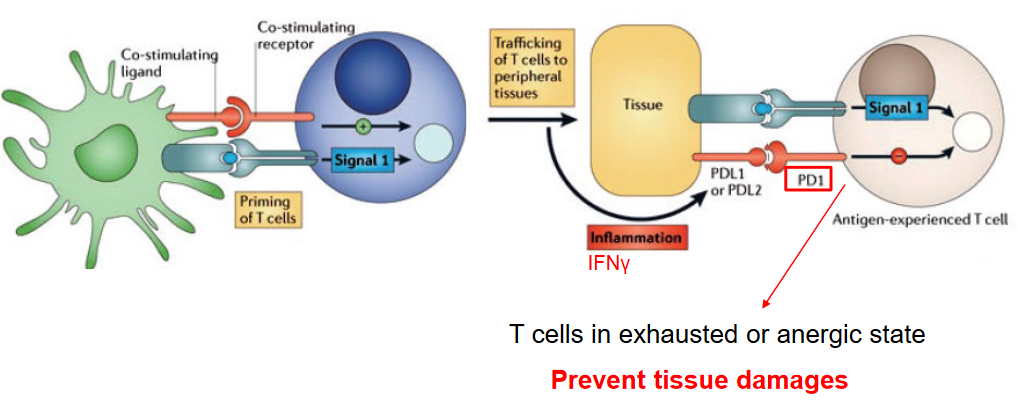
Programmed cell death protein 1 (PD-1) is a crucial inhibitory receptor expressed on the surface of T cells, playing a pivotal role in regulating immune responses and preventing tissue damage. PD-1 serves as a checkpoint molecule that helps maintain immune homeostasis by inhibiting T cell activation and preventing excessive immune responses.
When T cells encounter antigens, they undergo activation and proliferation to mount an immune response against pathogens or tumor cells. However, prolonged or excessive activation of T cells can lead to tissue damage and autoimmune reactions. PD-1 helps prevent such immune-mediated tissue damage by downregulating T cell activation and function under certain conditions.
In situations of chronic antigen exposure, such as persistent viral infections or cancer, T cells can become exhausted or anergic, losing their ability to mount effective immune responses. PD-1 is upregulated on these exhausted T cells as a mechanism to dampen their activity and prevent tissue damage caused by prolonged immune activation. By inducing T cell exhaustion or anergy, PD-1 helps maintain immune tolerance and prevents autoimmunity.
While PD-1 plays a critical role in preventing tissue damage and maintaining immune homeostasis, its overactivation can also contribute to immune evasion by tumors. Cancer cells often exploit the PD-1/PD-L1 pathway to evade immune surveillance and suppress anti-tumor immune responses. Blockade of PD-1 or its ligand PD-L1 has emerged as a promising strategy for cancer immunotherapy, aiming to unleash the anti-tumor immune response and enhance immune-mediated tumor clearance.
8.4.2.1 Immunotherapy Using Anti-PD-1 Antibodies
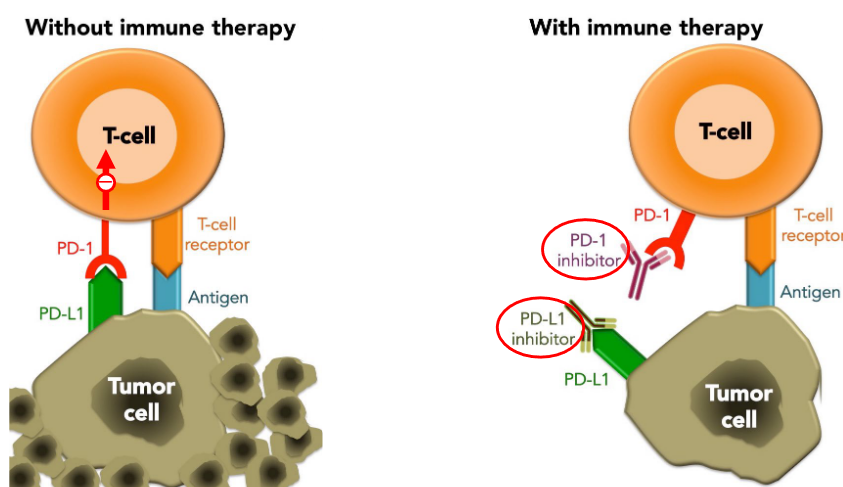
Immune therapy utilizing anti-PD-1/PD-L1 antibodies has revolutionized cancer treatment by harnessing the body’s immune system to combat tumors. PD-L1, often expressed on tumor cells, is frequently induced by interferon-gamma (IFN-γ) secreted from T helper 1 (Th1) cells. This interaction between PD-L1 and PD-1 leads to immune suppression, allowing tumors to evade detection and destruction by the immune system. However, anti-PD-1 or PD-L1 antibodies disrupt this inhibitory pathway, unleashing the immune system’s ability to recognize and attack cancer cells.
Clinical trials have demonstrated remarkable efficacy with at least a 52% response rate observed in various cancers treated with these therapies. Immune checkpoint blockade, achieved through the administration of anti-PD-1/PD-L1 antibodies, has shown significant success in extending survival and inducing durable responses in patients with advanced cancers.
In a significant milestone, the FDA approved the use of anti-PD-1/PD-L1 antibodies for treating melanomas in 2014, marking a paradigm shift in cancer therapy. Since then, these therapies have received approvals for a wide range of cancers, including lung cancer, bladder cancer, and Hodgkin’s lymphoma, among others.
8.5 CTLA4

Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is a critical inhibitory receptor expressed on the surface of T cells, primarily regulatory T cells (Tregs), but also on activated conventional T cells. It plays a crucial role in modulating immune responses and maintaining immune homeostasis. CTLA-4 serves as a checkpoint molecule that dampens T cell responses by inhibiting their activation and proliferation.
When T cells are activated through interaction with antigen-presenting cells (APCs) and co-stimulatory signals, CTLA-4 is upregulated on the surface of T cells. CTLA-4 competes with CD28, another co-stimulatory receptor on T cells, for binding to CD80/CD86 molecules on APCs. Unlike CD28, which promotes T cell activation, CTLA-4 delivers inhibitory signals that dampen T cell responses and prevent excessive immune activation.
By dampening T cell responses, CTLA-4 helps maintain consistent levels of T cell activation and prevents hyperactivation, which can lead to tissue damage and autoimmune reactions. This regulatory function of CTLA-4 is particularly important in preventing autoimmunity and maintaining immune tolerance to self-antigens.
Therapeutically, blockade of CTLA-4 has been explored as a strategy to enhance anti-tumor immune responses. By inhibiting CTLA-4, immune checkpoint inhibitors can unleash T cell activation and unleash the immune system’s ability to recognize and attack cancer cells. Drugs targeting CTLA-4, such as ipilimumab, have been approved for the treatment of advanced melanoma and have shown efficacy in other cancer types as well.
8.5.1 Anti-CTLA4 Antibodies for Immunotherapy

Immune therapy utilizing anti-CTLA-4 antibodies has emerged as a significant advancement in cancer treatment, particularly for melanomas. The FDA granted approval for this therapy in 2011, marking a significant milestone in the field of oncology. By targeting CTLA-4, these antibodies disrupt the inhibitory signals that dampen T cell responses, thereby unleashing the immune system’s ability to recognize and attack cancer cells. This approach has shown promise in extending survival and inducing durable responses in patients with advanced melanomas and other cancer types.
Concerning regulatory T cells (Tregs), CTLA-4 is constitutively expressed on their surface and plays a crucial role in their suppressive function. Tregs are a specialized subset of T cells that help maintain immune tolerance and prevent autoimmunity by suppressing the activation and proliferation of effector T cells. CTLA-4 expressed on Tregs contributes to their suppressive activity by delivering inhibitory signals that dampen immune responses.
In the context of anti-CTLA-4 antibody therapy, the blockade of CTLA-4 may disrupt the suppressive function of Tregs, leading to enhanced anti-tumor immune responses. By inhibiting CTLA-4 on Tregs, these antibodies may reduce the inhibitory signals delivered by Tregs, allowing effector T cells to mount more robust anti-tumor responses. This dual effect, targeting both effector T cells and Tregs, contributes to the efficacy of anti-CTLA-4 antibody therapy in promoting anti-tumor immunity.
8.6 Humanized Mouse Models
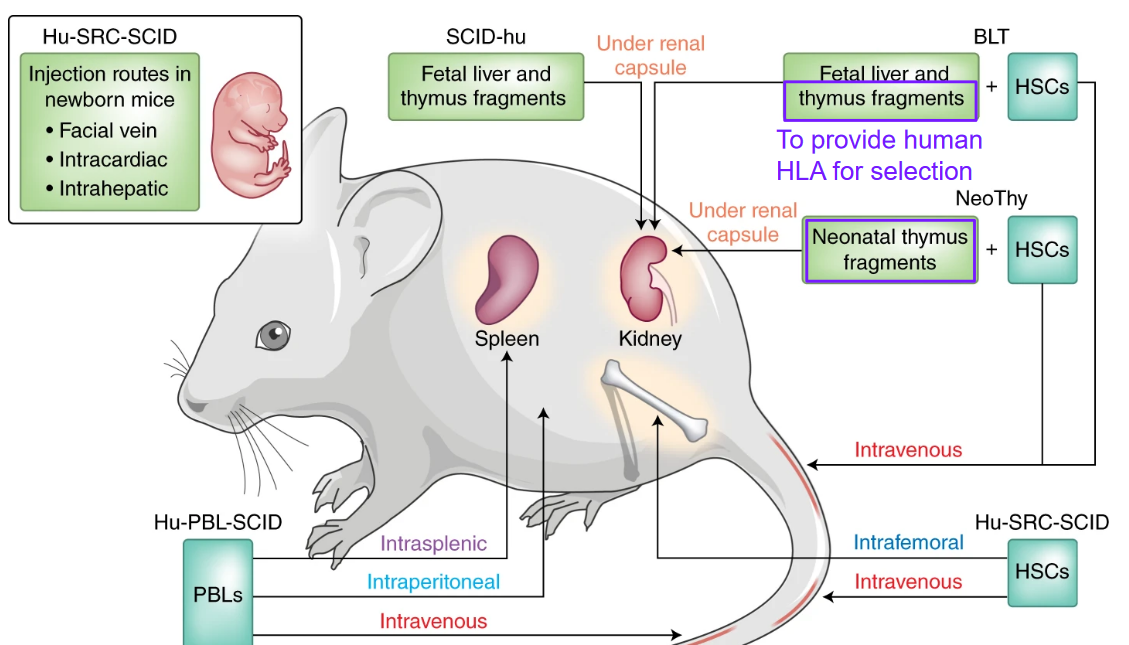
Humanized mouse models are valuable tools used in biomedical research to mimic human physiology and diseases in vivo. These models are created by introducing human cells, tissues, or genes into immunodeficient mice, allowing researchers to study human-specific processes, including immune responses and disease pathogenesis, in a living organism.
One essential component of humanized mouse models is the introduction of human leukocyte antigen (HLA) genes. HLA molecules are crucial for the presentation of antigens to T cells and play a central role in the adaptive immune response. By introducing human HLA genes into mouse models, researchers can generate mice that express human HLA molecules on their cells, closely resembling the human immune system.
8.7 Anti-Cancer T-Cell Responses
In addition to immune checkpoint blockade and CAR T-cell therapy, several other strategies can enhance anti-tumor immune responses by targeting various components of the immune system, including T cells, macrophages, and natural killer (NK) cells. These approaches aim to boost the immune system’s ability to recognize and eliminate cancer cells effectively.
One such strategy is adoptive cell therapy, which involves the infusion of ex vivo expanded tumor-specific T cells or NK cells into patients. These cells are engineered or selected to target specific tumor antigens, thereby enhancing anti-tumor immune responses and promoting tumor regression.
Another approach is cytokine therapy, where cytokines such as interleukin-2 (IL-2), interleukin-12 (IL-12), or interferon-alpha (IFN-α) are administered to stimulate the proliferation and activation of immune cells, including T cells, NK cells, and macrophages. These cytokines enhance effector functions and promote anti-tumor immunity.
Tumor vaccines represent another avenue for enhancing anti-tumor immune responses. These therapeutic vaccines contain tumor-specific antigens or peptides designed to stimulate the immune system to recognize and attack cancer cells. By activating T cells and inducing immune memory, tumor vaccines can lead to long-term anti-tumor responses.
8.7.1 CD19 CAR for B-Cell Lymphoma
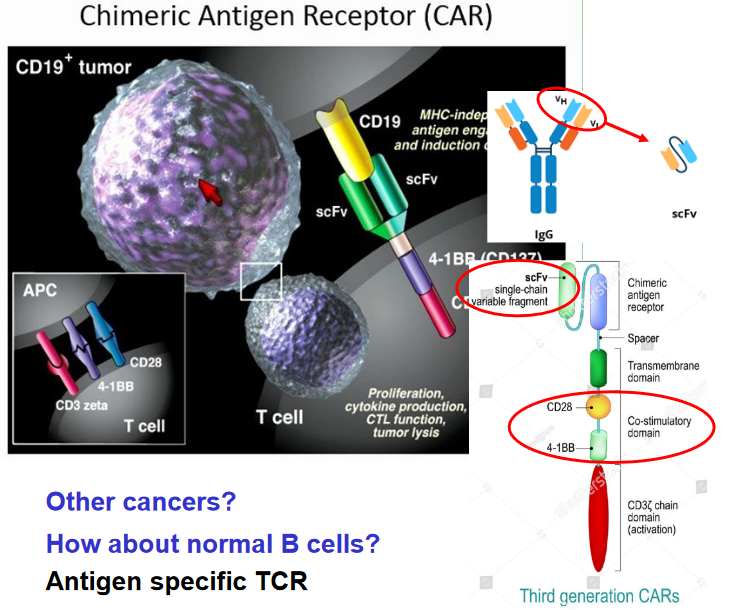
The process of utilizing CD19 CAR (Chimeric Antigen Receptor) therapy for treating B cell-derived lymphoma involves several key steps. Firstly, T cells are collected from the patient. These T cells are then genetically modified using a lentivirus to express the CD19 CAR. The CAR construct comprises an extracellular antibody domain that specifically binds to CD19, a protein found on the surface of B cells, and intracellular T cell signaling domains.
Once the T cells have been transduced to express the CAR, they are expanded ex vivo, meaning they are grown and multiplied outside the body in laboratory conditions. This expansion step increases the number of modified T cells available for treatment. After expansion, the modified T cells are reinfused back into the patient.
Upon reinfusion, the modified T cells target and bind to CD19-expressing B cells present in the patient’s body. The binding of the CAR to CD19 activates the intracellular signaling domains within the T cells, triggering a cytotoxic response against the B cells. This cytotoxic response leads to the destruction of the CD19-expressing B cells, thereby reducing the tumor burden.
Furthermore, the activated T cells undergo in vivo proliferation, meaning they multiply and expand within the patient’s body. This proliferation enhances the anti-tumor immune response, allowing the modified T cells to continue targeting and eliminating CD19-expressing B cells even after the initial infusion.
8.7.2 CD47

Disruption of the “don’t eat me” signal mediated by CD47 involves several mechanisms that promote the recognition and clearance of apoptotic cells by phagocytes. One such mechanism is the exposure of phosphatidylserine (PS) on the surface of apoptotic cells. PS serves as an “eat me” signal that facilitates the recognition and engulfment of apoptotic cells by phagocytes.
Additionally, components of the complement system, such as C1q, can opsonize apoptotic cells and promote their recognition and clearance by phagocytes. C1q binds to the surface of apoptotic cells, marking them for phagocytosis by immune cells.
Furthermore, calreticulin (CRT) is another molecule involved in disrupting the “don’t eat me” signal. CRT is normally localized within the endoplasmic reticulum, but during apoptosis, it translocates to the cell surface where it acts as an “eat me” signal for phagocytes.

The combination of an anti-CD47 antibody with rituximab demonstrates synergistic effects in promoting phagocytosis and eradicating non-Hodgkin lymphoma (NHL). CD47 is a “don’t eat me” signal expressed on the surface of cancer cells, including NHL cells, which prevents their recognition and clearance by phagocytes. Rituximab is a monoclonal antibody that targets CD20, a protein expressed on B cells, including NHL cells, leading to their destruction through various mechanisms, including antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).
When an anti-CD47 antibody is combined with rituximab, it disrupts the CD47-mediated inhibitory signal, thereby promoting the recognition and phagocytosis of NHL cells by phagocytes, such as macrophages. This synergistic effect enhances the efficacy of rituximab-mediated tumor cell destruction by augmenting the immune system’s ability to clear NHL cells.
8.8 NK Cells and Other Strategies
8.8.1 Balance Between Acivation and Inhibition

Natural killer (NK) cells play a crucial role in immune surveillance by identifying and eliminating infected or transformed cells. The balance between activating and inhibitory receptors on NK cells is essential for regulating their activity and ensuring effective immune responses while preventing autoimmunity.
Activating receptors on NK cells, such as NKG2D and DNAM-1, recognize stress-induced ligands or adhesion molecules expressed on target cells. Engagement of these activating receptors triggers NK cell activation and cytotoxicity, leading to target cell destruction.
In contrast, inhibitory receptors, such as killer cell immunoglobulin-like receptors (KIRs) and CD94/NKG2A, recognize self-major histocompatibility complex (MHC) class I molecules expressed on healthy cells. Binding of MHC class I molecules to inhibitory receptors delivers inhibitory signals that prevent NK cell activation and cytotoxicity against healthy cells.
The balance between activating and inhibitory signals determines NK cell activity. In healthy cells expressing sufficient levels of MHC class I molecules, inhibitory signals prevail, maintaining NK cell tolerance and preventing autoimmune responses. However, in infected or transformed cells with reduced MHC class I expression, activating signals dominate, leading to NK cell activation and target cell killing.
Imbalances in the expression or function of activating and inhibitory receptors can dysregulate NK cell activity, contributing to immune dysfunction and disease pathogenesis. For example, downregulation of MHC class I molecules on tumor cells can evade NK cell recognition, leading to immune escape and tumor progression. Conversely, overactivation of NK cells due to insufficient inhibitory signals can result in autoimmune diseases or tissue damage.
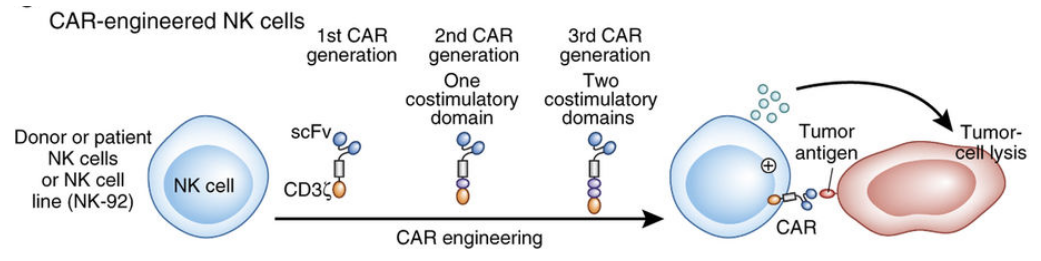
Tipping the balance towards enhancing activating signals in natural killer (NK) cells represents a promising strategy in immunotherapy to bolster immune responses against pathogens and cancer. Various approaches can be employed to achieve this goal. One avenue involves targeted antibody therapy, where monoclonal antibodies are designed to bind specific activating receptors on NK cells, such as NKG2D or DNAM-1, thereby stimulating their signaling pathways and augmenting NK cell activation and cytotoxicity against target cells. Additionally, cytokine therapy offers another means of boosting NK cell function by administering cytokines like interleukin-2 (IL-2), interleukin-12 (IL-12), or interleukin-15 (IL-15) to promote NK cell activation and expansion.
NK cell engagers, such as bispecific antibodies or antibody-derived molecules, can be engineered to simultaneously bind activating receptors on NK cells and tumor antigens on target cells, triggering NK cell activation and target cell killing independently of major histocompatibility complex (MHC) recognition. Furthermore, inhibitors targeting negative regulators of NK cell function, such as checkpoint molecules like PD-1 or TIGIT, can unleash NK cell activity by blocking inhibitory signals. Finally, genetic engineering techniques can be employed to enhance NK cell function, including the introduction of chimeric antigen receptors (CARs) to redirect NK cell specificity towards tumor antigens, thereby enhancing their killing activity.
8.8.2 Stem Cell Transportation

Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a vital treatment option for acute myeloid leukemia (AML), particularly for patients who are at high risk of relapse or have achieved remission but are at risk of disease recurrence. This procedure involves replacing the patient’s diseased bone marrow with healthy hematopoietic stem cells obtained from a genetically matched donor, typically a sibling or unrelated donor.
The success of allo-HSCT in AML is attributed to the graft-versus-leukemia (GVL) effect, where the donor immune cells recognize and eliminate residual leukemia cells, thereby reducing the risk of disease relapse. Additionally, the conditioning regimen administered before transplant, which typically includes chemotherapy and/or radiation therapy, helps to eradicate any remaining leukemia cells and create space for the donor stem cells to engraft.
Despite its efficacy, allo-HSCT is associated with significant risks, including graft-versus-host disease (GVHD), where the donor immune cells attack the recipient’s tissues, and transplant-related complications such as infection and organ toxicity. Therefore, patient selection, donor selection, and careful management of post-transplant complications are crucial for optimizing outcomes.
Recent advancements in allo-HSCT, such as reduced-intensity conditioning regimens and improved supportive care measures, have expanded the eligibility and safety of transplantation for a broader range of AML patients, including older adults and those with comorbidities. Furthermore, novel strategies, such as haploidentical transplantation and post-transplant maintenance therapies, are being investigated to further enhance the efficacy and reduce the toxicity of allo-HSCT in AML.
8.8.3 iNKT and MAIT Cells

Natural Killer T (NKT) cells are a subset of T cells that express markers found on both T cells and natural killer cells. Two distinct populations of NKT cells are the Invariant NKT (iNKT) cells and Mucosal-Associated Invariant T (MAIT) cells.
iNKT cells are characterized by their expression of a semi-invariant T cell receptor (TCR) that recognizes glycolipid antigens presented by the non-classical major histocompatibility complex (MHC) molecule CD1d. These cells play crucial roles in bridging the innate and adaptive immune responses and are known for their rapid cytokine production upon activation. iNKT cells are involved in immune regulation, inflammation, and tumor surveillance.
MAIT cells, on the other hand, are a subset of T cells that recognize microbial vitamin B metabolites presented by the non-classical MHC class I-related molecule MR1. MAIT cells are abundant in mucosal tissues, such as the gut and lungs, where they play essential roles in immune defense against bacterial, viral, and fungal infections. MAIT cells are particularly well-known for their rapid response to microbial antigens and their involvement in maintaining mucosal homeostasis.
Both iNKT cells and MAIT cells exhibit innate-like properties, including rapid activation, cytokine production, and effector functions, making them critical components of the early immune response against pathogens.
8.8.3.1 DC Maturation by iNKT Cells
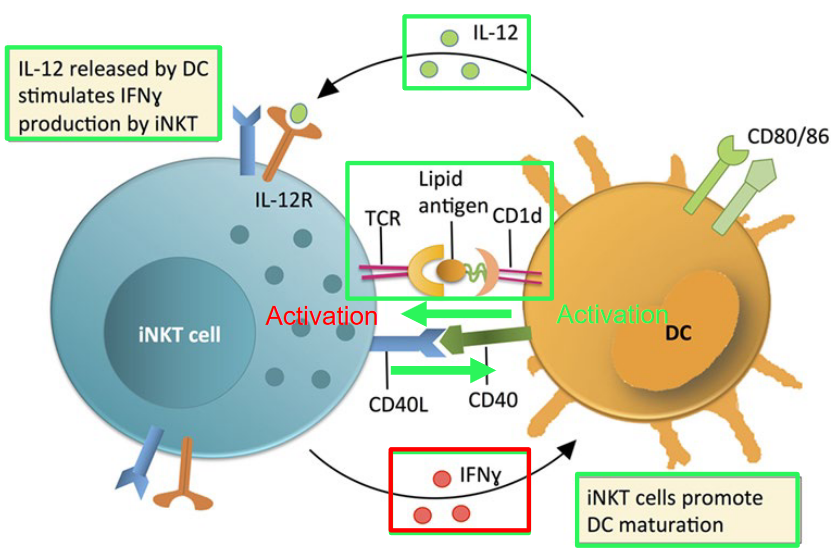
Invariant Natural Killer T (iNKT) cells, when activated by dendritic cells (DCs), play a significant role in promoting DC maturation. This process enhances the ability of DCs to present antigens and stimulate other immune cells, thus contributing to an effective immune response against pathogens or tumor cells. Additionally, a subset of iNKT cells expressing CD4 can produce Th2-related cytokines, such as interleukin-4 (IL-4) and interleukin-13 (IL-13). These cytokines play a crucial role in regulating immune responses, particularly in promoting allergic and anti-parasitic responses.
In mice, iNKT cells can be classified based on the expression of surface markers CD4 and CD8 into distinct subsets. CD4+ iNKT cells are capable of expressing a higher level of Th2-related cytokines compared to their CD4- counterparts, indicating their potential to skew immune responses towards a Th2 phenotype. Conversely, the CD4- iNKT cell subset, also known as double-negative (DN) iNKT cells, may have distinct functional properties and cytokine profiles, contributing to a diverse range of immune responses.
8.8.4 \(\gamma\delta\) T-Cells

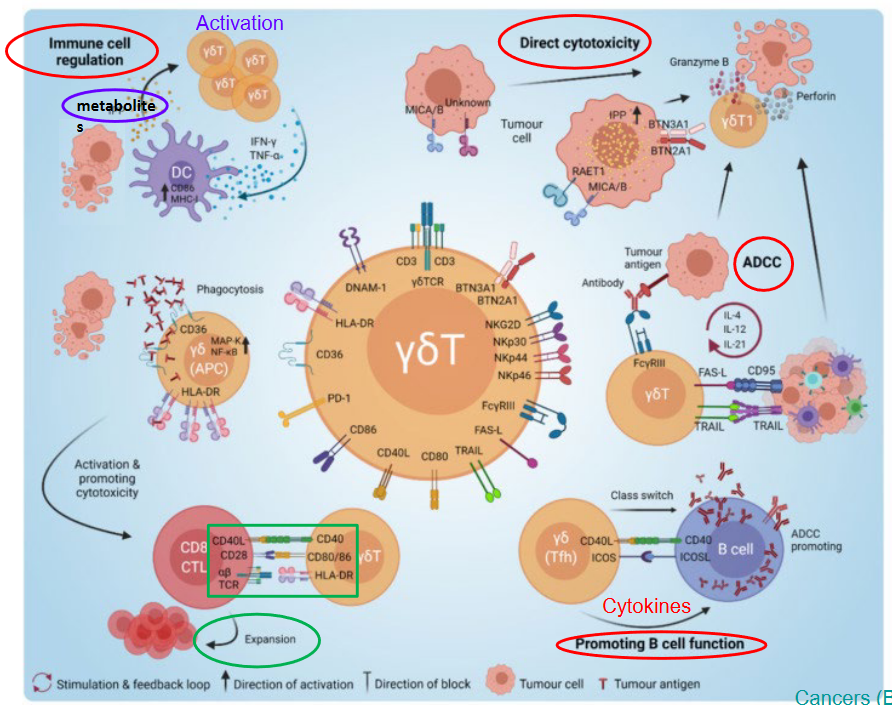
Gamma-delta (γδ) T cells, a subset of T cells characterized by the expression of a T cell receptor (TCR) composed of γ and δ chains, exhibit diverse functions in the context of tumor immunity. These functions can be broadly categorized into anti-tumor and pro-tumor roles.
Anti-tumor functions of γδ T cells: 1. Cytotoxicity: γδ T cells possess potent cytotoxic capabilities, allowing them to directly kill tumor cells through the release of cytotoxic molecules such as perforin and granzyme. 2. Tumor cell recognition: γδ T cells can recognize and target tumor cells independent of major histocompatibility complex (MHC) molecules. They recognize stress-induced molecules or antigens presented by non-classical MHC molecules expressed on tumor cells. 3. Production of cytokines: Upon activation, γδ T cells produce cytokines such as interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), which contribute to anti-tumor immune responses by promoting inflammation, enhancing antigen presentation, and activating other immune cells.
Pro-tumor functions of γδ T cells: 1. Immunoregulation: Some subsets of γδ T cells exhibit immunosuppressive properties by producing regulatory cytokines such as interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β). These cytokines can suppress anti-tumor immune responses and promote tumor growth. 2. Tumor-promoting inflammation: In certain contexts, γδ T cells may contribute to tumor progression by promoting chronic inflammation within the tumor microenvironment. This chronic inflammation can facilitate tumor growth, angiogenesis, and metastasis. 3. Induction of immune tolerance: γδ T cells may participate in the induction of immune tolerance, allowing tumors to evade immune surveillance and establish immune privilege within the tumor microenvironment.