
7 Immune Responses
7.1 Bone Marrow Transplantation
Bone marrow transplantation is a medical procedure used to treat problems with the immune system caused by issues in how white blood cells, called lymphocytes, develop and work. These issues can make it hard for the body to fight off infections.
During the transplant, doctors replace unhealthy bone marrow, which produces these important white blood cells, with healthy bone marrow from a donor. This new bone marrow contains cells that can develop into healthy lymphocytes, helping to boost the immune system and improve the body’s ability to fight infections.
This procedure can be especially helpful for people with certain types of immunodeficiency disorders where their immune system isn’t working properly. By replacing the faulty bone marrow with healthy bone marrow, doctors aim to restore normal immune function and improve the patient’s overall health.
7.1.1 Problems
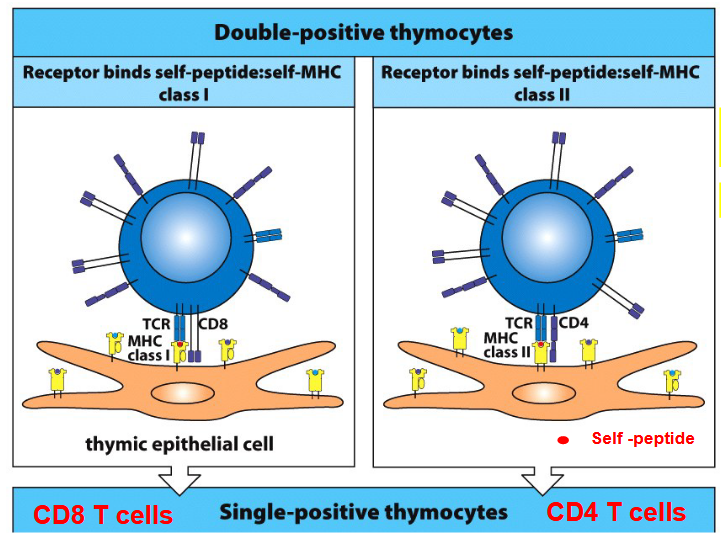
After bone marrow transplantation, the T cells produced from the transplanted stem cells encounter another obstacle. These T cell precursors undergo a process of selection in the thymus, which is an important organ for immune system development.
Thymic epithelial cells play a crucial role in this process. Through positive selection, these cells present self-peptides along with major histocompatibility complex (MHC) molecules to developing T cells. This helps in selecting T cells that can recognize these self-peptides in the context of MHC, ensuring they can function effectively while avoiding attacking the body’s own cells.
However, in the case of bone marrow transplantation, where the donor and recipient may have different MHC molecules, a challenge arises. The recipient’s cortical thymic epithelial cells (cTECs) may positively select T cells that recognize self-MHC, while the donor’s MHC, which the T cells will encounter in the body, is not positively selected.
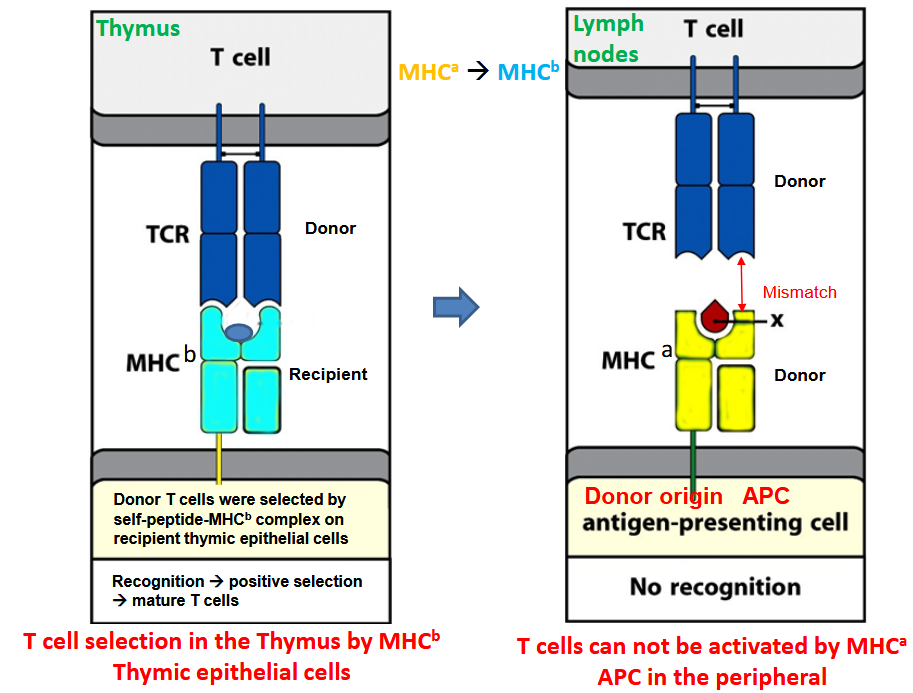
This discrepancy leads to a problem where the antigen-presenting cells (APCs) in the body are of donor origin, potentially causing the T cells to respond too strongly, leading to cell death. To address this, there’s a process of negative selection, where medullary thymic epithelial cells (mTECs) and dendritic cells (DCs) from the donor present self-peptides along with donor MHC molecules.

Cells that recognize these self-peptides weakly undergo conventional positive selection, becoming mature T cells. Those that react strongly are eliminated to prevent autoimmune responses. Some T cells with intermediate strength reactions may become regulatory T cells (Tregs), which help in controlling immune responses
7.2 Gene Therapy
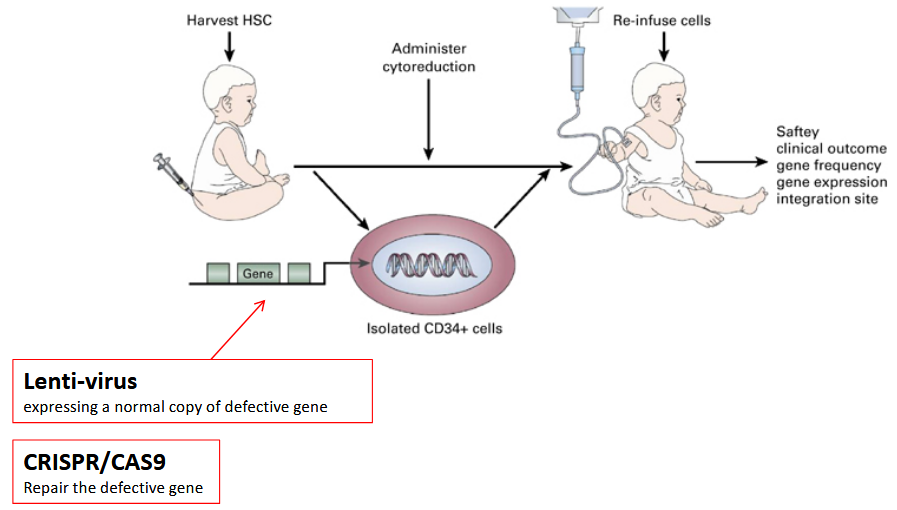
Gene therapy utilizing self-hematopoietic stem cells involves modifying the genetic material of these stem cells to correct or treat genetic disorders or diseases.
Hematopoietic stem cells are unique because they can give rise to various types of blood cells, including red blood cells, white blood cells, and platelets. By introducing therapeutic genes into these stem cells, either through viral vectors or other delivery mechanisms, researchers aim to address genetic abnormalities that cause diseases such as sickle cell anemia, hemophilia, or certain types of immunodeficiency disorders.
Once the modified stem cells are reintroduced into the patient’s body, they can potentially produce healthy blood cells carrying the corrected genetic information, offering a promising approach for long-term treatment of these conditions.
7.3 Phases of Infection

Firstly, there’s the exposure phase, where the pathogen first enters the body, often through inhalation, ingestion, or contact with mucous membranes or broken skin. Following exposure, the pathogen begins to replicate and spread, leading to the incubation phase, during which the individual may not yet show symptoms but the pathogen is multiplying within the body.
Once the pathogen reaches a critical mass, symptoms begin to appear, marking the onset of the symptomatic phase. This phase varies depending on the type of pathogen and the individual’s immune response, but symptoms often include fever, fatigue, coughing, sneezing, and other signs of illness.
As the immune system recognizes the infection, it mounts a response, leading to the immune response phase. During this phase, immune cells such as white blood cells and antibodies work to identify and neutralize the pathogen, often resulting in the resolution of symptoms as the infection is cleared.
However, in some cases, particularly with persistent or virulent pathogens, the infection may progress to the chronic phase, where symptoms persist over an extended period, or the infection may become latent, with the pathogen remaining dormant within the body.
7.3.1 T-Cell Receptors

TLRs, or Toll-like receptors, are an essential part of the immune system, recognizing various molecules from pathogens and activating immune responses. In most cases, the signaling pathways initiated by TLR activation rely on a protein called MyD88. MyD88 acts as a crucial mediator in transmitting signals from TLRs to downstream molecules that trigger inflammatory responses and immune defenses. However, there is an exception to this rule: TLR3.
Unlike the majority of TLRs, TLR3 utilizes a signaling pathway that is independent of MyD88. Instead, TLR3 activates a different pathway involving a protein called TRIF.
7.3.1.1 Innate Immune System and the Adaptive Immune System
The innate immune response serves as a crucial initial line of defense against invading pathogens and is essential for triggering and shaping the adaptive immune response. When the body encounters a pathogen, cells of the innate immune system, such as macrophages, dendritic cells, and natural killer cells, recognize and respond to the threat by initiating inflammatory responses, phagocytosis, and the release of signaling molecules called cytokines. These innate immune responses help contain and eliminate the pathogen, creating an environment conducive to the activation of the adaptive immune system.
The adaptive immune response, characterized by the actions of T and B lymphocytes, is highly specific to the particular pathogen encountered. However, the activation of adaptive immunity relies on signals provided by the innate immune system. Antigen-presenting cells, such as dendritic cells, play a critical role in bridging the innate and adaptive immune responses by presenting pathogen-derived antigens to T cells. This presentation, along with co-stimulatory signals provided by the innate immune cells, activates T cells and initiates the adaptive immune response.
Furthermore, certain components of the innate immune system, such as Toll-like receptors (TLRs), are involved in recognizing pathogen-associated molecular patterns (PAMPs) and initiating signaling cascades that lead to both innate and adaptive immune responses. In this way, the innate immune system acts as a sentinel, alerting and priming the adaptive immune system to respond effectively to specific pathogens.
7.3.1.2 Changing CD4+ T Cell Differentiation

Cytokines, which are small proteins involved in cell signaling, play a significant role in determining the differentiation of CD4 T cells into various subsets with distinct functions. The differentiation of CD4 T cells is crucial for orchestrating effective immune responses against pathogens, as different subsets of CD4 T cells have specialized roles in regulating immune reactions. Manipulation of cytokines can profoundly influence this process, leading to changes in CD4 T cell differentiation.
For example, the cytokine interleukin-12 (IL-12) promotes the differentiation of CD4 T cells into T helper 1 (Th1) cells. Th1 cells are important for combating intracellular pathogens such as viruses and certain bacteria. On the other hand, cytokines such as interleukin-4 (IL-4) and interleukin-6 (IL-6) can induce the differentiation of CD4 T cells into T helper 2 (Th2) cells. Th2 cells are involved in mediating immune responses against extracellular parasites and in allergic reactions.
Additionally, transforming growth factor-beta (TGF-β) and interleukin-6 (IL-6) can drive the differentiation of CD4 T cells into T helper 17 (Th17) cells. Th17 cells are important for protecting mucosal surfaces from bacterial and fungal infections. Furthermore, TGF-β in combination with interleukin-2 (IL-2) can induce the differentiation of regulatory T (Treg) cells, which play a crucial role in maintaining immune tolerance and preventing autoimmune diseases.
7.4 T-Cells
7.4.1 Th1 Cells

The effector function of T helper 1 (Th1) cells is primarily focused on combating intracellular pathogens, such as viruses and certain bacteria, through various mechanisms. When Th1 cells are activated, they secrete cytokines, particularly interferon-gamma (IFN-γ), which plays a central role in coordinating the immune response against intracellular pathogens.
IFN-γ has several effects that help in controlling intracellular infections. Firstly, it activates macrophages, stimulating them to become more efficient at phagocytosis and killing of engulfed pathogens. This process, known as macrophage activation, enhances the ability of macrophages to eliminate intracellular pathogens.
Additionally, IFN-γ promotes the differentiation of CD8 cytotoxic T cells, which are essential for directly killing infected cells. CD8 T cells recognize and destroy cells that have been infected with intracellular pathogens, thereby limiting the spread of the infection.
Furthermore, Th1 cells can also induce the production of antibodies, particularly IgG2a in mice (or IgG1 in humans), which can facilitate the elimination of intracellular pathogens by enhancing phagocytosis and activating complement-mediated lysis.
7.4.2 Th2 Cells

The effector function of T helper 2 (Th2) cells is primarily geared towards combating extracellular parasites, such as helminths (worms) and certain protozoa, through specialized immune responses. When Th2 cells become activated, they secrete cytokines that orchestrate the immune system’s response to these types of pathogens.
One key cytokine secreted by Th2 cells is interleukin-4 (IL-4). IL-4 has several effects that are particularly effective against extracellular parasites. Firstly, IL-4 promotes the production of antibodies, particularly immunoglobulin E (IgE) antibodies. IgE antibodies play a crucial role in defense against extracellular parasites by binding to their surfaces and marking them for destruction by other components of the immune system, such as eosinophils.
In addition to antibody production, Th2 cells also stimulate the recruitment and activation of eosinophils, which are specialized white blood cells capable of releasing toxic proteins to kill parasites. Eosinophils are especially effective against helminth parasites and are often found at sites of infection.
Furthermore, Th2 cells can induce the production of mucus and other secretions that help trap and expel parasites from mucosal surfaces, such as the gastrointestinal tract and respiratory tract. This process, known as mucosal immunity, provides an additional barrier against extracellular parasites.
7.4.2.1 B-Cells?
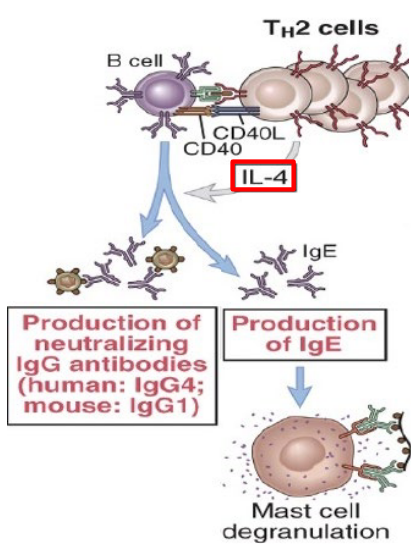
When Th2 cells become activated in response to antigens from extracellular parasites, they release cytokines such as interleukin-4 (IL-4) and interleukin-13 (IL-13). These cytokines play a pivotal role in stimulating B cells to undergo class switching to produce IgE antibodies. Unlike other antibody classes like IgG and IgM, which are effective against various types of pathogens, IgE antibodies are specifically tailored to combat extracellular parasites.
Once produced, IgE antibodies bind to receptors on the surface of mast cells and basophils, which are types of immune cells found in tissues throughout the body. This binding sensitizes these cells to the presence of the parasite antigen. Upon subsequent encounters with the parasite, the antigen binds to the IgE antibodies bound to the mast cells or basophils, triggering the release of inflammatory mediators such as histamine and leukotrienes.
Additionally, Th2 cells indirectly support B cell activation and antibody production by promoting the differentiation of follicular helper T cells (Tfh). Tfh cells migrate to germinal centers within lymphoid tissues, where they interact with B cells undergoing proliferation and antibody maturation
7.4.3 Th17 Cells

When Th17 cells become activated in response to signals from dendritic cells and other immune cells, they secrete cytokines such as interleukin-17 (IL-17) and interleukin-22 (IL-22). These cytokines play critical roles in recruiting and activating various immune cells to fight against extracellular bacteria and fungi.
One of the primary functions of Th17 cells is to promote the recruitment of neutrophils to sites of infection. Neutrophils are highly effective at engulfing and destroying extracellular bacteria through a process called phagocytosis. IL-17 produced by Th17 cells stimulates the production of chemokines and adhesion molecules by local tissue cells, which attract neutrophils to the site of infection, enhancing their ability to eliminate the invading pathogens.
Additionally, Th17 cells can induce the production of antimicrobial peptides by epithelial cells and other barrier tissues. These antimicrobial peptides have direct bactericidal and fungicidal properties, providing an additional layer of defense against extracellular pathogens.
Furthermore, Th17 cells contribute to tissue repair and maintenance of barrier integrity following infection. IL-22, produced by Th17 cells, promotes the proliferation and differentiation of epithelial cells, enhancing their ability to form physical barriers and preventing the spread of pathogens into deeper tissues.
7.4.4 Different T-Cell Subsets Inhibit Development of One Another
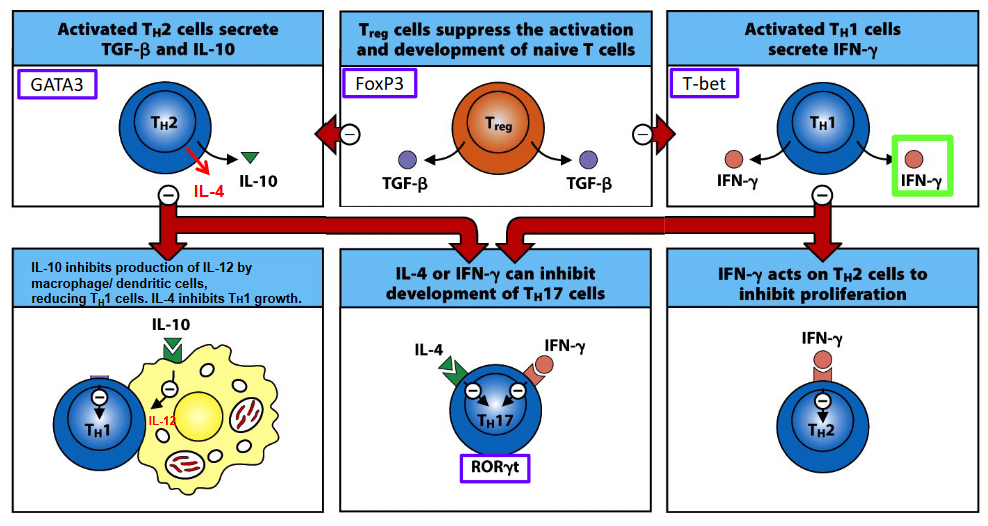
One example of T cell subset regulation is the reciprocal inhibition between T helper 1 (Th1) and T helper 2 (Th2) cells. Th1 cells, which primarily combat intracellular pathogens, produce cytokines such as interferon-gamma (IFN-γ) that can inhibit the development of Th2 cells. Conversely, Th2 cells, which are involved in responses against extracellular parasites, can suppress Th1 cell development through cytokines like interleukin-4 (IL-4) and interleukin-10 (IL-10).
Similarly, regulatory T cells (Tregs), a subset known for their immunosuppressive functions, can inhibit the development and activation of other T cell subsets. Tregs produce inhibitory cytokines such as interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β) and express cell surface molecules like cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), which can suppress the activity of effector T cells, including Th1, Th2, and Th17 cells.
Moreover, T helper 17 (Th17) cells, which are involved in responses against extracellular bacteria and fungi, can reciprocally inhibit the development of Tregs through mechanisms that involve cytokines like IL-17 and IL-23.
7.4.5 Central and Effector Memory T Cells
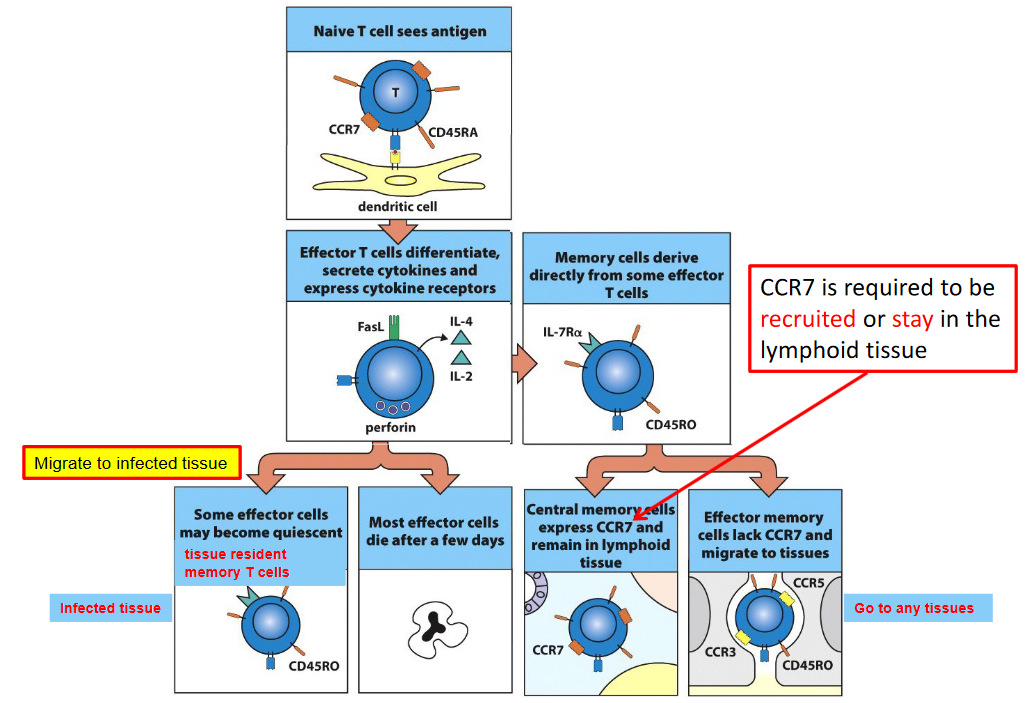
Central memory T cells (Tcm) and effector memory T cells (Tem) circulate between the lymphoid tissues and peripheral tissues, while tissue-resident memory T cells (Trm) reside in peripheral tissues, providing rapid and localized immune responses upon re-exposure to antigens.
CCR7, a chemokine receptor, plays a crucial role in the recruitment and retention of T cells in lymphoid tissues. Central memory T cells express high levels of CCR7, allowing them to migrate to and reside within secondary lymphoid organs, such as lymph nodes and spleen. In contrast, effector memory T cells exhibit reduced expression of CCR7 and preferentially traffic to non-lymphoid tissues, where they survey for pathogens and respond rapidly to infections.
Tissue-resident memory T cells, on the other hand, are specialized subsets that establish residence within peripheral tissues, such as the skin, mucosa, and other barrier sites, following previous encounters with pathogens. Unlike central and effector memory T cells, tissue-resident memory T cells do not require CCR7 for recruitment or retention in lymphoid tissues. Instead, they express distinct homing receptors that facilitate their localization and retention within peripheral tissues, where they serve as sentinels, providing immediate and site-specific immune protection upon antigen re-encounter.
7.4.6 CD4 and CD8 T-Cells
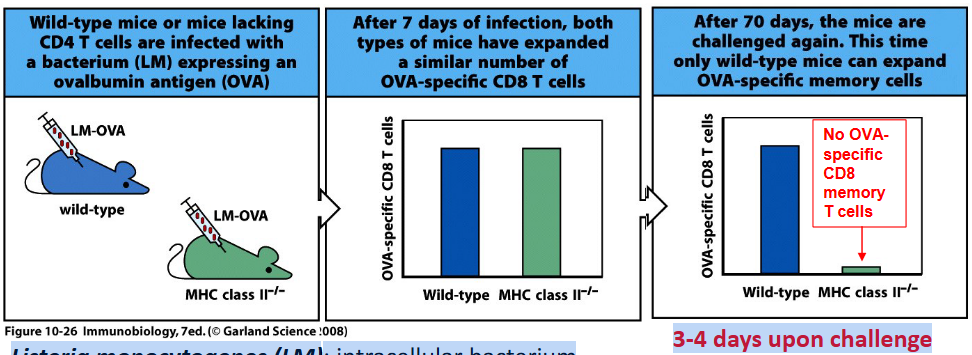
Deficiency in genes related to MHC class II antigen presentation, such as those encoding MHC class II molecules or components of the antigen processing and presentation pathway, will result in a lack of CD4+ T cell population. This is because MHC class II molecules are essential for presenting antigens to CD4+ T cells, which in turn stimulate the activation and differentiation of CD4+ T cells into various effector and memory subsets.
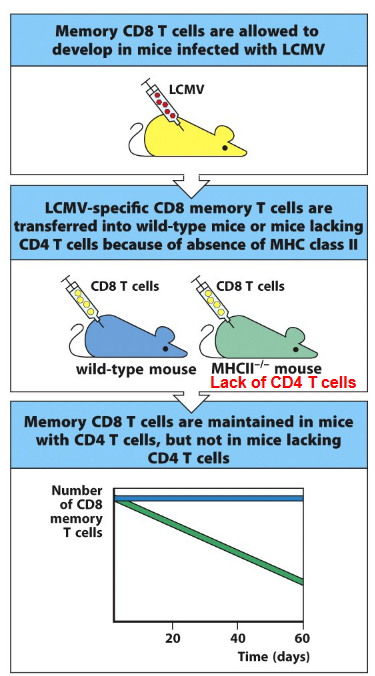
The requirement of CD4 T cells for the generation of CD8 memory T cells has been demonstrated through experimental studies. One approach involves depleting CD4 T cells before immunization with an antigen and subsequent infection. Researchers have observed that in the absence of CD4 T cells, the generation of CD8 memory T cells is impaired. This impairment is evident in reduced numbers and functionality of memory CD8 T cells upon re-exposure to the antigen.
Moreover, studies using genetically modified mice lacking CD4 T cells or MHC class II molecules have provided further evidence for the requirement of CD4 T cells in CD8 memory T cell generation. These mice exhibit deficiencies in CD8 memory T cell responses compared to wild-type mice when challenged with pathogens or antigens.
Additionally, adoptive transfer experiments, where CD4 T cells from immunized donors are transferred into naive recipients, have shown that CD4 T cells play a crucial role in promoting the generation and maintenance of CD8 memory T cells. Conversely, depletion of CD4 T cells after immunization results in impaired development of CD8 memory T cell responses.
7.4.6.1 Maintenance of CD8 T-Cells
One key mechanism by which CD4 T cells support CD8 memory T cell maintenance is through the provision of help signals during the priming phase. Upon encountering antigens, CD4 T cells provide essential co-stimulatory signals and cytokines, such as interleukin-2 (IL-2) and interleukin-21 (IL-21), which are crucial for the activation, proliferation, and differentiation of CD8 T cells into memory cells. These signals not only facilitate the initial generation of CD8 memory T cells but also imprint them with the ability to persist long-term.
Furthermore, CD4 T cells continue to provide support to CD8 memory T cells during the memory phase. Through the secretion of cytokines like IL-2 and IL-21, CD4 T cells help sustain the survival and functionality of CD8 memory T cells. IL-2, in particular, has been shown to enhance the survival and homeostatic proliferation of memory CD8 T cells. Moreover, CD4 T cells can provide signals that help maintain the expression of key molecules involved in CD8 memory T cell function, such as the IL-7 receptor (IL-7R), which is crucial for the homeostatic maintenance of memory T cells.
Experimental studies have also demonstrated the importance of CD4 T cells in maintaining CD8 memory T cell responses. Depletion of CD4 T cells after the establishment of CD8 memory T cell populations results in a decline in their numbers and functionality, highlighting the ongoing dependency of CD8 memory T cells on CD4 T cell help for their maintenance.
7.5 Dendritic Cells
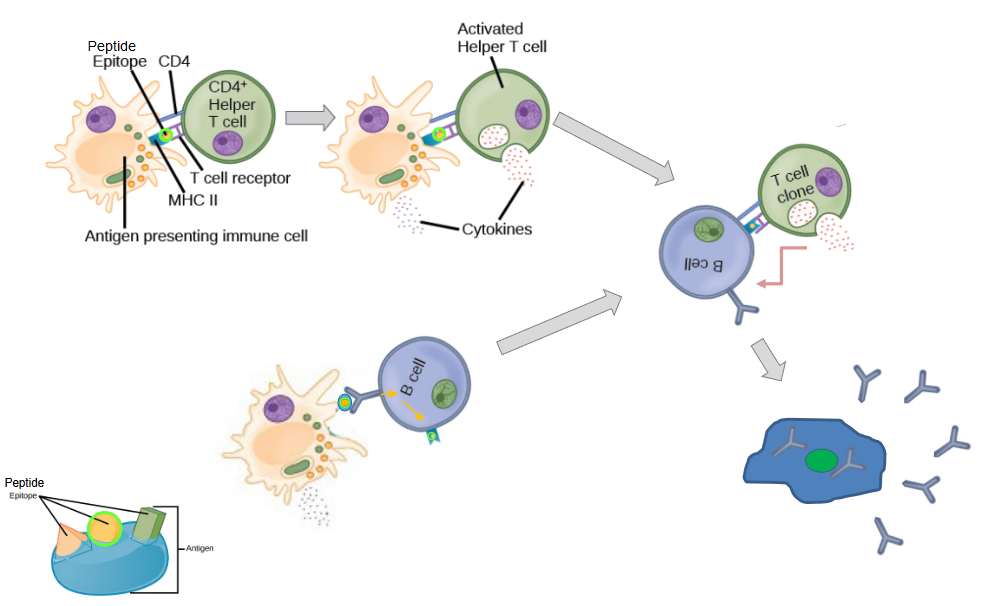
When dendritic cells encounter antigens, such as those derived from pathogens or vaccines, they engulf these antigens and process them into smaller fragments. Dendritic cells then migrate to secondary lymphoid organs, such as lymph nodes, where they present these antigen fragments to CD4+ T cells and B cells.
For CD4+ T cell activation, dendritic cells act as professional antigen-presenting cells (APCs). They present antigen fragments on their cell surface in the context of major histocompatibility complex class II (MHC II) molecules. This interaction between dendritic cells and CD4+ T cells is essential for initiating T cell responses. Dendritic cells provide co-stimulatory signals to CD4+ T cells, which, together with the antigen presentation, lead to the activation and proliferation of specific subsets of CD4+ T cells, such as T helper cells. These activated CD4+ T cells can then differentiate into effector cells, such as Th1, Th2, Th17, or T follicular helper cells, depending on the cytokine milieu and co-stimulatory signals provided by dendritic cells.
In addition to activating CD4+ T cells, dendritic cells also play a critical role in activating B cells. Dendritic cells can present antigens to B cells directly or indirectly through the presentation of antigen fragments in the context of MHC II molecules. This interaction provides crucial signals for B cell activation, including antigen recognition and co-stimulation. Upon activation, B cells proliferate and differentiate into antibody-secreting plasma cells or memory B cells. Plasma cells produce antibodies specific to the antigen encountered, while memory B cells provide long-term immunity by quickly mounting an immune response upon re-exposure to the antigen.
7.6 Types of Inteferons
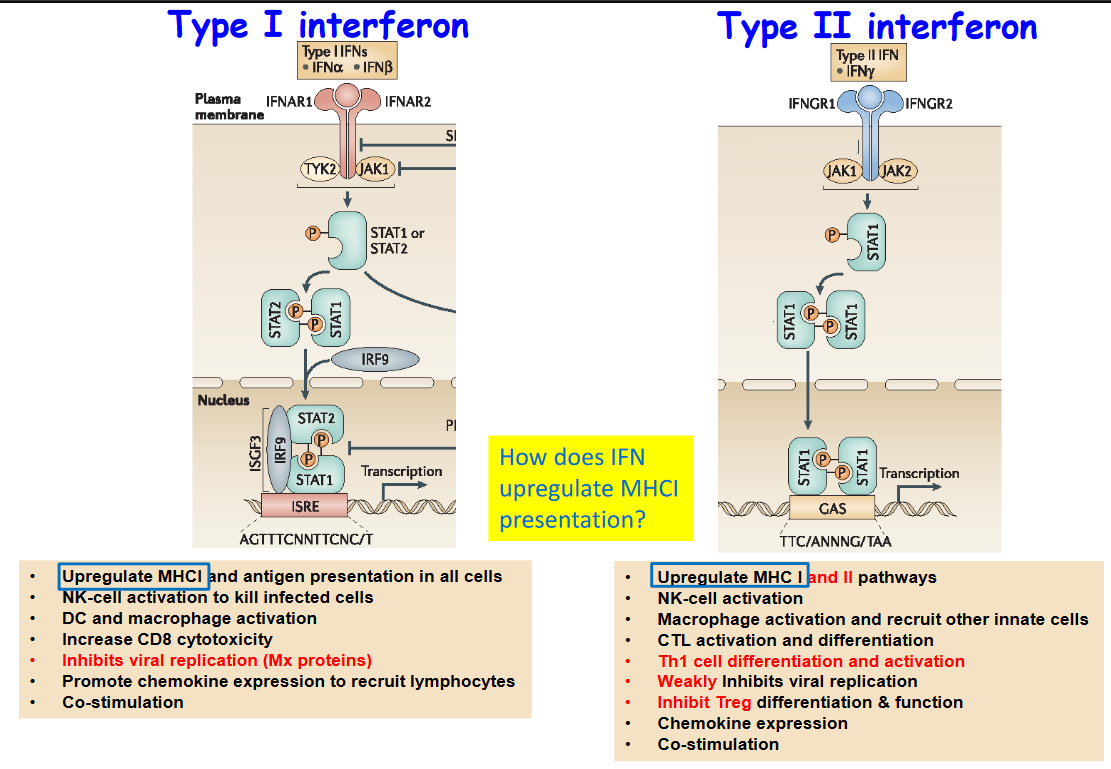
Interferons are essential proteins produced by cells in response to viral infections and other immune challenges.
Type I interferons play a multifaceted role in immune defense by boosting antigen presentation across all cells, activating natural killer (NK) cells to eliminate infected cells, and enhancing the activity of dendritic cells (DCs) and macrophages, vital for engulfing and presenting antigens to T cells. Additionally, Type I interferons increase the cytotoxicity of CD8+ T cells, inhibit viral replication within infected cells through the induction of antiviral proteins, and promote the recruitment of immune cells like lymphocytes to sites of infection by inducing the expression of chemokines. Moreover, Type I interferons provide co-stimulatory signals to activate various immune cells, further bolstering the antiviral response.
On the other hand, Type II interferon, also known as interferon-gamma, contributes to immune defense by upregulating the expression of major histocompatibility complex (MHC) molecules on cell surfaces, thereby enhancing antigen presentation. It also activates NK cells, which play a pivotal role in eliminating virus-infected cells, and boosts the activity of macrophages while recruiting other innate immune cells to the site of infection. Additionally, Type II interferon aids in the activation and differentiation of cytotoxic T lymphocytes (CTLs) and T helper 1 (Th1) cells, both crucial for orchestrating effective antiviral immune responses. Though its direct inhibition of viral replication is weaker compared to Type I interferons, Type II interferon inhibits the differentiation and function of regulatory T cells (Tregs), further promoting antiviral immunity. Lastly, it contributes to the expression of chemokines and provides co-stimulatory signals to bolster immune activation against viral threats.
7.7 Immunoproteasomes
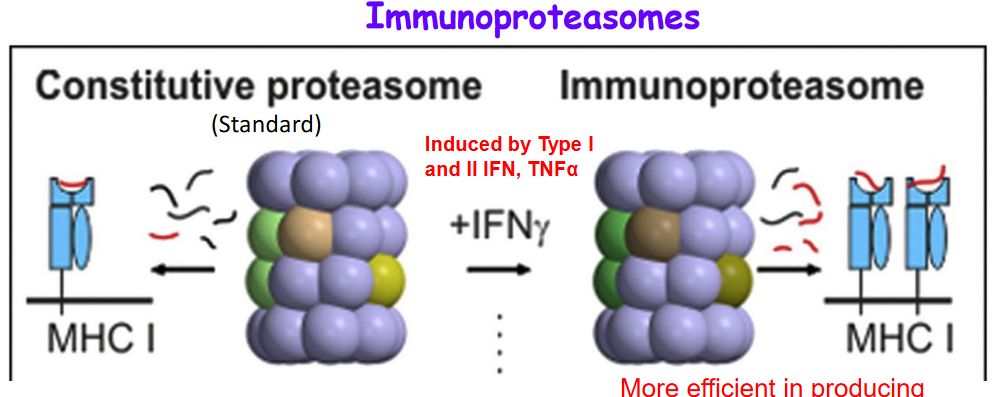
Immunoproteasomes are specialized protein complexes found in cells that play a crucial role in generating peptides for loading onto major histocompatibility complex class I (MHC-I) molecules. These peptides are then presented on the cell surface, where they can be recognized by CD8+ T cells as part of immune surveillance against infections and abnormal cells. Immunoproteasomes are more efficient than standard proteasomes in producing peptides that are better suited for MHC-I loading, making them particularly adept at generating immunogenic peptides. They are induced by various signals, including Type I and Type II interferons (IFNs) and tumor necrosis factor-alpha (TNF-α), highlighting their importance in the immune response against infections and cancer. The peptides produced by immunoproteasomes are recognized by primed CD8+ T cells, which are poised to mount an immune response against specific antigens, further highlighting the significance of immunoproteasomes in adaptive immunity.
7.7.1 Not All Cells Express an Immunoproteasome

Not all cells express immunoproteasomes because their expression is tightly regulated and often induced by specific signals, such as Type I and Type II interferons (IFNs) and tumor necrosis factor-alpha (TNF-α). The majority of cells typically express standard proteasomes, which are the default form of proteasomes in most tissues.
Interestingly, in tumors, there is a predominance of standard proteasomes rather than immunoproteasomes. This could be due to several reasons. One potential explanation is that tumors may downregulate the expression of immunoproteasomes as part of their immune evasion strategies, as immunoproteasome expression may enhance antigen presentation and immune recognition, which could hinder tumor growth. Additionally, the tumor microenvironment may not provide the necessary signals, such as IFNs, to induce immunoproteasome expression.
In normal tissues, the predominant expression of standard proteasomes serves a protective role. This is because standard proteasomes produce peptides that are less immunogenic compared to those generated by immunoproteasomes. By expressing standard proteasomes, tissues can avoid being targeted by primed CD8+ T cells in the absence of inflammation. This helps prevent unnecessary immune responses and tissue damage under normal physiological conditions.
However, in response to inflammatory signals, such as IFNs, somatic cells have the ability to replace standard proteasomes with immunoproteasomes. This allows cells to enhance their antigen presentation capabilities and mount more robust immune responses against infections or other threats
7.8 B Cells
7.8.1 Memory B Cell Generation
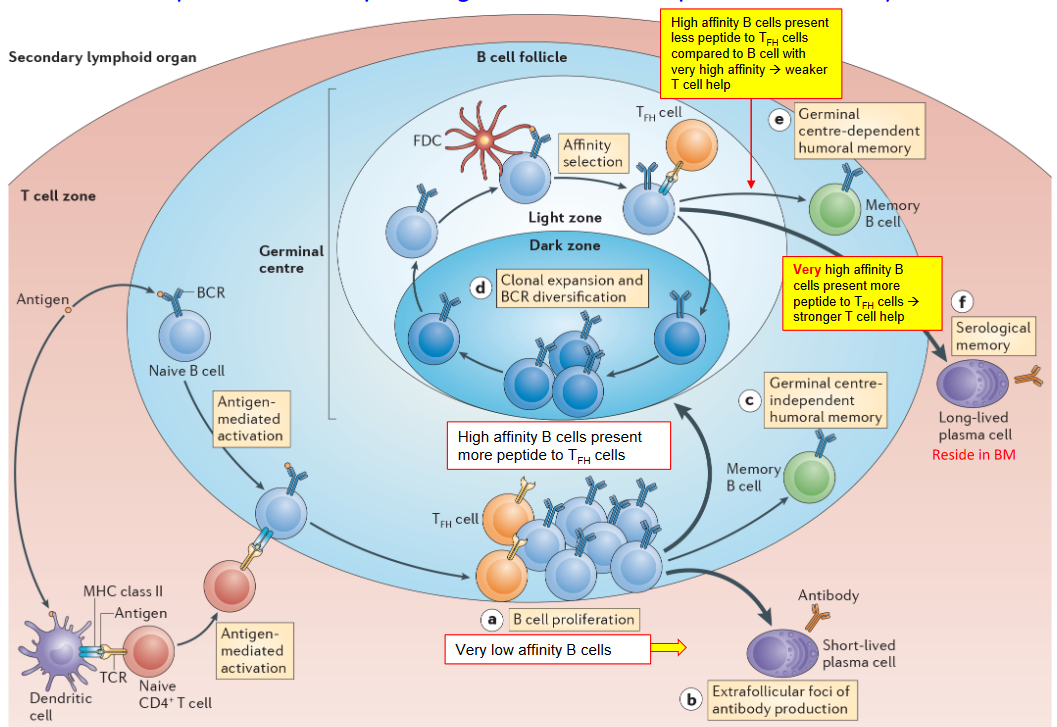
Germinal center B cells undergo a rigorous selection process to differentiate into memory B cells, which play a crucial role in providing long-term immunity upon re-exposure to pathogens. One primary mechanism for the selection of germinal center B cells as memory B cells involves affinity for the antigen and T cell help.
During the germinal center reaction, B cells with higher affinity for the antigen receive more signals from T follicular helper (Tfh) cells, which provide critical help for B cell activation and differentiation. B cells that successfully capture antigens and present them to Tfh cells receive stronger co-stimulatory signals and cytokine support, leading to their selection as memory B cells. This affinity-based selection ensures that memory B cells have a higher likelihood of recognizing and responding to the pathogen upon subsequent encounters.
In addition to affinity and T cell help, several other mechanisms contribute to the selection of germinal center B cells as memory B cells. One such mechanism involves the forced expression of pro-survival factors, which promotes the survival and longevity of memory B cells, thus expanding the memory B cell pool.
Furthermore, the downregulation of the transcription factor Bach2 is associated with the differentiation of germinal center B cells into plasma cells rather than memory B cells. Bach2 downregulation is controlled by various signals, including very strong T cell help combined with high affinity B cell receptor (BCR) signaling. This results in the differentiation of germinal center B cells into plasma cells, which are specialized for antibody secretion rather than memory formation.
Additionally, the expression of transcription factors such as Blimp1 and IRF4 promotes the differentiation of germinal center B cells into plasma cells. These transcription factors drive the expression of genes required for plasma cell development and antibody production.
7.8.2 Different Signalling Capacities
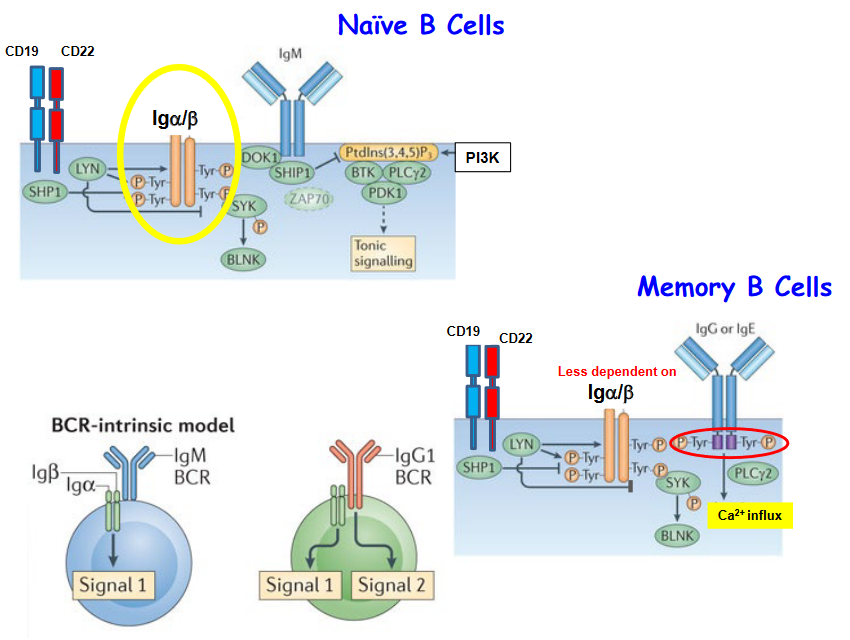
The signaling capacity between IgM and IgG B cell receptors (BCRs) differs notably, impacting the responses of memory B cells and naive B cells. One significant distinction lies in the downstream signaling pathways activated upon BCR engagement, including calcium (Ca2+) influx and phosphoinositide 3-kinase (PI3K) signaling.
Upon antigen binding, IgM BCRs typically trigger robust Ca2+ influx and PI3K activation in B cells. These signaling events are crucial for initiating B cell activation, proliferation, and differentiation. In contrast, IgG BCRs often induce weaker Ca2+ influx and PI3K signaling compared to IgM BCRs. This differential signaling capacity may result from variations in the structural properties and downstream signaling molecules associated with IgM and IgG BCRs.
Additionally, memory B cells, which predominantly express IgG BCRs, exhibit less dependency on Ca2+ influx and PI3K signaling compared to naive B cells, which primarily express IgM BCRs. Memory B cells, having undergone previous antigen exposure and activation, may possess altered signaling thresholds and enhanced sensitivity to antigen re-stimulation. As a result, memory B cells can mount rapid and efficient responses even with reduced reliance on certain signaling pathways.
In contrast, naive B cells require strong and sustained signaling through pathways such as Ca2+ influx and PI3K activation to initiate robust immune responses upon encountering antigens for the first time.
7.9 Immunological Memory
Memory B cells and memory T cells are the primary mediators of immunological memory. These cells persist in the body after the resolution of an infection or vaccination, ready to mount a rapid and robust immune response upon re-exposure to the same pathogen or antigen. Memory B cells can produce high-affinity antibodies more quickly and in larger quantities than naive B cells, while memory T cells exhibit enhanced effector functions and a faster response time compared to naive T cells.
For some infections and vaccinations, immunological memory can last for decades, providing long-term protection against recurrent infections. For example, individuals who have been vaccinated against diseases such as measles, mumps, and rubella often retain protective immunity for many years, if not for their entire lives. Similarly, memory T cells generated during primary infections with certain pathogens, such as influenza virus or cytomegalovirus, can persist for decades and confer protection against subsequent infections.
However, the duration of immunological memory is not uniform for all pathogens and antigens. Some infections or vaccinations may induce relatively short-lived memory responses, requiring booster vaccinations to maintain protective immunity over time. Additionally, aging and other factors that affect immune function can influence the longevity of immunological memory.
7.9.1 Antibody Production

One significant factor influencing the longevity of antibody protection is the nature of the pathogen or antigen encountered. Some infections induce long-lasting immunity, with antibodies persisting for years or even a lifetime. For example, individuals who recover from measles or chickenpox typically develop long-term immunity due to the production of memory B cells and antibodies that provide lasting protection against reinfection.
In contrast, antibody protection against certain pathogens may not be lifelong. One contributing factor is the ability of pathogens to undergo antigenic variation, altering their surface antigens to evade pre-existing immunity. This phenomenon is evident in infections like influenza and HIV, where new strains emerge over time, potentially reducing the effectiveness of previously generated antibodies. Additionally, the decline in antibody levels over time, particularly in the absence of ongoing exposure to the pathogen, can lead to a waning of immunity and increased susceptibility to reinfection.
Age-related changes in the immune system, known as immunosenescence, can also affect the duration of antibody protection. Older individuals may experience a decline in immune function, including the production and maintenance of antibodies, which can impact the effectiveness and longevity of immune responses. Furthermore, the characteristics of vaccines, such as the type of vaccine and the presence of adjuvants or booster doses, can influence the duration of antibody protection conferred by immunization.
7.9.2 Original Antigenic Sin (i.e., OAS)

One disadvantage of immunological memory, particularly in the context of the antibody response and viral antigen-specific CD8 T cells, is a phenomenon known as “original antigenic sin.” This phenomenon occurs when the immune system is exposed to a new strain or variant of a virus that shares similarities with a previously encountered strain. In such cases, the immune response may preferentially target epitopes from the previous strain, leading to a suboptimal or skewed immune response against the new virus.
The problem arises because the immune system relies on memory B cells and memory T cells generated during previous infections or vaccinations to recognize and respond to subsequent encounters with the same pathogen. However, if the new virus possesses antigenic features that resemble those of the previous virus strain, the immune response may be biased towards epitopes from the original strain. As a result, the production of antibodies and activation of CD8 T cells may be primarily directed against epitopes from the previous strain, rather than effectively neutralizing the new virus.
This phenomenon can be particularly problematic in the context of rapidly evolving viruses, such as influenza virus or HIV, where new strains emerge regularly through genetic mutations or antigenic drift. In such cases, the presence of original antigenic sin may limit the effectiveness of the immune response against newly emerging viral variants, as the immune system may struggle to mount a robust and targeted response against the novel antigens presented by the new virus.
7.9.3 Antibody-Dependent Enhancement (i.e., ACE)

Antibody-dependent enhancement (ADE) is a phenomenon in which antibodies, instead of neutralizing the virus, facilitate viral entry into host cells, leading to increased viral replication and disease severity. ADE can occur when the levels or quality of antibodies produced in response to a viral infection or vaccination are insufficient to completely neutralize the virus. This inadequacy may arise due to factors such as low antibody concentration or low antibody avidity, which refers to the strength of binding between antibodies and viral antigens.
In cases of ADE, antibodies bind to the surface of the virus but fail to effectively neutralize it. Instead, the virus-antibody complex is internalized into host cells via receptor-mediated endocytosis. Once inside the cell, the virus can evade lysosomal degradation, a process that would typically occur in the absence of antibodies. As a result, the virus can replicate within the host cell, leading to increased viral load and potentially exacerbating the severity of the infection.
ADE has been observed in several viral infections, including dengue virus, Zika virus, and coronaviruses such as SARS-CoV and MERS-CoV. In these cases, suboptimal antibody responses or cross-reactive antibodies generated in response to previous infections or vaccinations may contribute to ADE. The phenomenon poses challenges for vaccine development and therapeutic interventions, as it highlights the importance of carefully evaluating antibody responses to ensure that they effectively neutralize the virus without promoting ADE.
To mitigate the risk of ADE, researchers and vaccine developers strive to design vaccines that induce robust and protective antibody responses against viral pathogens. This may involve selecting vaccine antigens that elicit broadly neutralizing antibodies, optimizing vaccine formulations and dosing regimens, and conducting thorough preclinical and clinical evaluations to assess the safety and efficacy of candidate vaccines.
7.9.3.1 Antibody Concentration and Avidity Matter

Firstly, antibody concentration refers to the abundance of antibodies present in the blood or other bodily fluids. Higher antibody concentrations generally correlate with increased neutralization of pathogens. When pathogens enter the body, antibodies with high concentrations can bind to and neutralize the pathogen more efficiently, preventing its entry into host cells and inhibiting its ability to replicate. Therefore, a sufficient concentration of antibodies is essential for robust immune protection against pathogens.
Secondly, antibody avidity refers to the strength and stability of the binding interaction between antibodies and their target antigens. High avidity antibodies have strong and durable interactions with antigens, whereas low avidity antibodies have weaker and less stable binding. Antibodies with higher avidity are more effective at neutralizing pathogens because they can bind more tightly to the pathogen’s surface proteins, preventing their interactions with host cells and enhancing their clearance from the body.
In the context of antibody-dependent enhancement (ADE), both antibody concentration and avidity are critical factors. Inadequate antibody concentrations or low avidity antibodies may contribute to ADE by allowing the virus-antibody complexes to enter host cells via receptor-mediated endocytosis. Once inside the cell, the virus can exploit host cellular machinery for replication, leading to increased viral load and exacerbation of the infection.
7.9.3.2 For SARS-CoV-1?
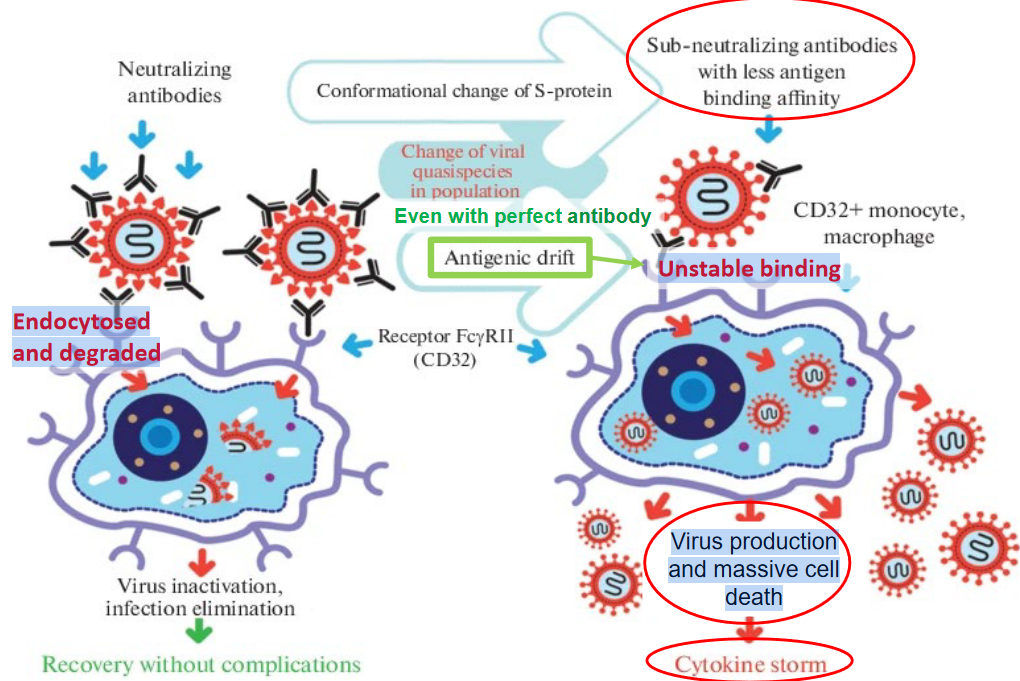
Antibody-dependent enhancement (ADE) has been observed in the context of severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) infections, particularly in animal models and in vitro studies. ADE occurs when suboptimal antibodies, instead of neutralizing the virus, facilitate viral entry into host cells, leading to increased viral replication and exacerbation of the disease. In the case of SARS-CoV-1, ADE can result in enhanced virus production and massive cell death, contributing to the severity of the infection.
One mechanism by which ADE may occur in SARS-CoV-1 infections involves the endocytosis of virus-antibody complexes into host cells. Antibodies that bind to the virus but fail to effectively neutralize it may form complexes that are internalized into host cells via receptor-mediated endocytosis. Once inside the cell, the virus can exploit host cellular machinery to replicate, leading to increased viral load and dissemination.
In the context of SARS-CoV-1, it has been suggested that ADE may be associated with unstable binding interactions between antibodies and viral antigens. Antibodies with low avidity or weak binding affinities may form complexes with the virus that are prone to dissociation or instability. These unstable complexes may still be recognized by cellular receptors and internalized into host cells, facilitating viral entry and replication.
7.9.4 Evading Immune Responses
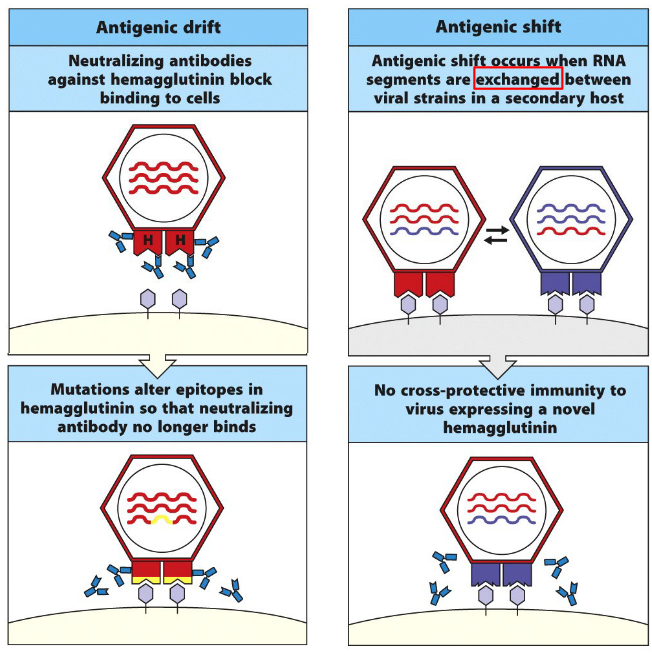
One common mechanism by which pathogens evade immune detection is through antigenic variation. This involves altering the surface proteins or antigens that are recognized by the immune system, thereby escaping recognition by antibodies and T cells. Pathogens such as influenza virus, HIV, and Plasmodium spp. (the causative agents of malaria) are known for their ability to undergo antigenic variation, enabling them to evade immune responses and establish chronic infections.
Another strategy employed by pathogens is the suppression or modulation of host immune responses. Pathogens may produce virulence factors or proteins that interfere with host immune signaling pathways, dampening the immune response and promoting immune evasion. For example, certain bacteria secrete toxins that inhibit phagocytosis or interfere with cytokine signaling, thereby impairing the host’s ability to mount an effective immune response. Similarly, viruses may encode proteins that antagonize key components of the host immune system, such as interferons or complement proteins, allowing the virus to replicate and spread unchecked.
Furthermore, pathogens can exploit host immune cells to establish persistent infections. Some viruses, such as herpesviruses and retroviruses, have evolved mechanisms to establish latent infections within host cells, evading immune detection by remaining dormant until conditions are favorable for reactivation. Additionally, pathogens may infect immune cells themselves, impairing their function and compromising the host’s ability to mount an effective immune response.
7.9.5 Molecular Mimicry and Autoimmunity
Molecular mimicry refers to a phenomenon in which foreign antigens, such as those derived from pathogens like bacteria or viruses, share structural similarities with self-antigens found in the host. This similarity can lead to cross-reactivity, where the immune system mistakenly targets host tissues and cells, resulting in autoimmune reactions. One notable example of the association between molecular mimicry and autoimmunity is seen in Guillain-Barré syndrome (GBS).
Guillain-Barré syndrome is an autoimmune disorder characterized by muscle weakness and, in severe cases, paralysis. It is believed that GBS can be triggered by molecular mimicry between certain microbial antigens, particularly those from bacteria like Campylobacter jejuni, and self-antigens found in peripheral nerves. When the immune system mounts a response against the invading pathogen, antibodies or T cells generated against microbial antigens may cross-react with nerve tissue, leading to inflammation and damage to peripheral nerves.
In the case of GBS, molecular mimicry between specific epitopes of C. jejuni lipooligosaccharides (LOS) and gangliosides present on peripheral nerves has been proposed as a potential mechanism. Gangliosides are complex molecules found on the surface of nerve cells, and antibodies produced against LOS during C. jejuni infection may cross-react with gangliosides, leading to autoimmune attack and nerve damage.
7.10 Vaccines
7.10.1 Killed Vaccines
7.10.1.1 Killed
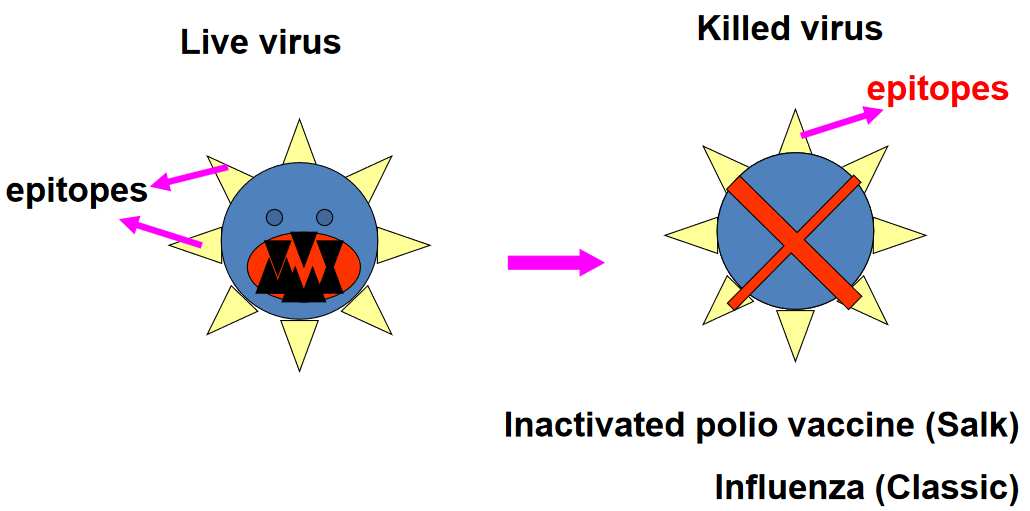

Killed vaccines are a type of vaccine that contains whole, inactivated forms of the pathogen they are designed to protect against. In the case of killed vaccines, the pathogen is typically killed using heat or chemical agents such as formaldehyde. This process renders the pathogen non-infectious while preserving its structural and antigenic components, allowing the immune system to recognize and mount a response against them without causing disease.
The use of heat or formaldehyde to inactivate the pathogen ensures that it cannot replicate or cause infection upon administration. This safety measure is essential for vaccine development, as it prevents the risk of disease transmission or adverse reactions associated with live pathogens. Once administered, the whole agent vaccine stimulates the immune system to produce antibodies and activate immune cells specific to the pathogen’s antigens, providing protection against future infections.
Examples of whole agent vaccines include the inactivated poliovirus vaccine (IPV), which contains killed poliovirus strains, and the inactivated influenza vaccine (flu shot), which contains inactivated influenza viruses. These vaccines have been instrumental in controlling and preventing infectious diseases, contributing to significant reductions in morbidity and mortality worldwide.
While whole agent vaccines are effective at inducing immune responses and providing protection against pathogens, they may require booster doses to maintain immunity over time. Additionally, some whole agent vaccines may induce milder immune responses compared to live attenuated vaccines, necessitating the use of adjuvants or multiple doses to enhance immunogenicity.
7.10.2 Live Attenuated
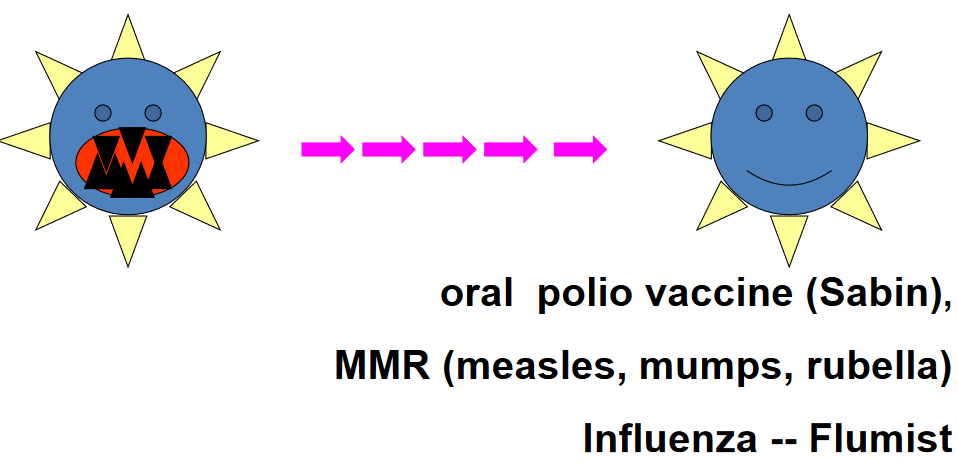
In this approach, the pathogen is weakened or attenuated through a process that reduces its virulence, or ability to cause disease, while retaining its ability to stimulate an immune response. Live attenuated vaccines mimic natural infections, leading to robust and long-lasting immune responses without causing disease.
The attenuation process can involve various methods, including serial passage of the pathogen in cell culture under suboptimal conditions, genetic manipulation to delete or modify virulence genes, or passage through non-human hosts. These methods result in mutations or changes to the pathogen’s genome that decrease its ability to replicate or cause disease in humans, while still allowing it to replicate sufficiently to induce an immune response.
Live attenuated vaccines offer several advantages over other types of vaccines. They typically require fewer doses to induce immunity, and they often provide broader and longer-lasting protection compared to inactivated vaccines. Additionally, live attenuated vaccines stimulate both cellular and humoral immune responses, providing comprehensive immunity against the target pathogen.
Examples of live attenuated vaccines include the measles, mumps, and rubella (MMR) vaccine, which contains weakened forms of the measles, mumps, and rubella viruses, and the oral poliovirus vaccine (OPV), which contains attenuated poliovirus strains. These vaccines have been highly effective in preventing their respective diseases and have contributed significantly to public health efforts worldwide.
Despite their benefits, live attenuated vaccines may pose risks to individuals with weakened immune systems, as the attenuated pathogen can potentially cause disease in these individuals. Additionally, the attenuation process must be carefully controlled to ensure that the vaccine strain maintains its weakened properties and does not revert to a virulent form.
7.10.3 Immune Memory
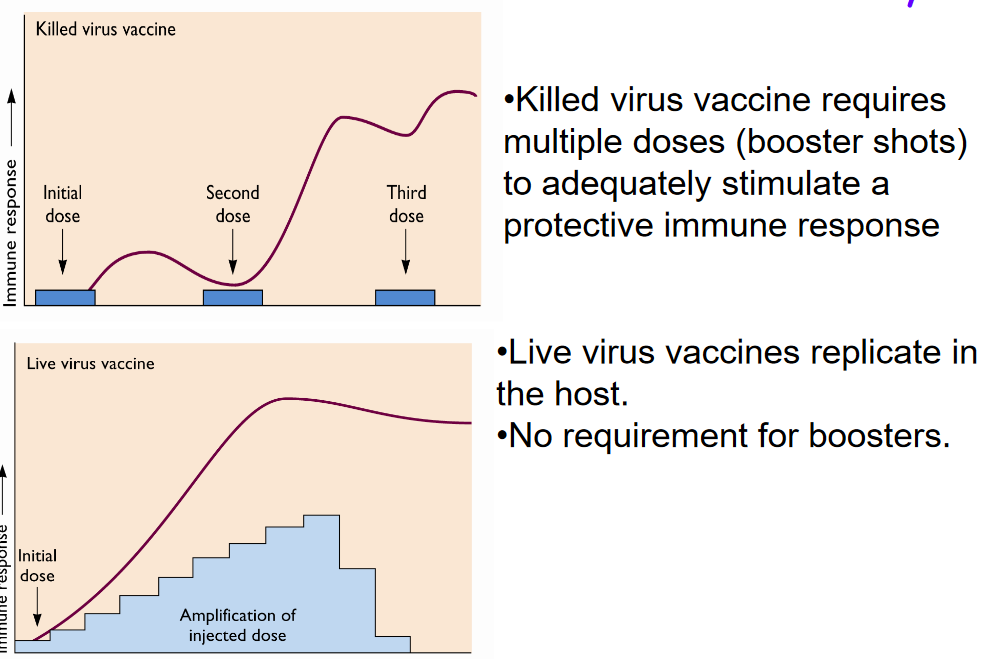
Vaccines play a crucial role in stimulating immune memory, which enables the immune system to recognize and respond rapidly to pathogens upon subsequent exposures, providing long-term protection against infectious diseases. However, different types of vaccines may vary in their ability to induce and maintain immune memory.
Killed virus vaccines, which contain inactivated forms of the pathogen, typically require multiple doses, also known as booster shots, to adequately stimulate a protective immune response. These vaccines may initially induce a weaker immune response compared to live virus vaccines because the inactivated pathogens cannot replicate in the host. As a result, additional doses are needed to reinforce and sustain the immune memory, ensuring robust and lasting protection against the target pathogen.
In contrast, live virus vaccines contain weakened or attenuated forms of the pathogen that can replicate in the host. Because live virus vaccines mimic natural infections more closely, they often induce stronger and more durable immune responses compared to killed virus vaccines. The replication of the attenuated virus within the host stimulates both cellular and humoral immune responses, leading to the generation of robust immune memory. As a result, live virus vaccines typically do not require booster shots to maintain immunity, as the initial vaccine dose is sufficient to establish long-lasting protection against the targeted disease.
Examples of live virus vaccines include the measles, mumps, and rubella (MMR) vaccine and the oral poliovirus vaccine (OPV). These vaccines have been highly effective in preventing their respective diseases and provide lifelong immunity with a single dose.
7.10.4 Constructing a Recombinant Attenuated Virus
Here is an outline of the general process:
Isolation of the Virus: The first step involves isolating the target virus from a clinical specimen or a laboratory culture. This provides the starting material for further genetic manipulation.
Cloning of the Genome: The genome of the isolated virus is then cloned into a suitable vector, such as a plasmid or a bacterial artificial chromosome (BAC). Cloning allows researchers to manipulate the viral genome in a controlled manner.
Identification and Isolation of Virulence Genes: Specific genes responsible for the virulence of the virus are identified and isolated. These genes are typically involved in key aspects of the virus’s ability to replicate, evade the immune system, or cause disease.
Mutation or Deletion of Virulence Genes: Using genetic engineering techniques such as site-directed mutagenesis or gene deletion, the virulence genes are modified to reduce or eliminate their function. This step aims to render the virus attenuated, meaning it is no longer capable of causing disease while retaining its ability to replicate and induce an immune response.
Verification of Attenuation: The resulting recombinant virus is tested to ensure that it meets the desired criteria: it should be viable (able to replicate), immunogenic (able to stimulate an immune response), and non-virulent (not capable of causing disease). Extensive testing is conducted to confirm that the attenuated virus is safe and effective for use as a vaccine.
In some cases, instead of directly attenuating the target virus, researchers may express viral antigens in a less harmful virus, such as an adenovirus vector. This approach, known as viral vector vaccines, allows for the delivery of viral antigens without the risk of causing disease. Adenovirus vectors, for example, are often used to express antigens from pathogens such as influenza virus, HIV, or SARS-CoV-2, providing a safe and efficient platform for vaccine development.
7.10.5 Subunit Vaccines

Subunit vaccines represent a type of vaccine that contains purified components or fragments of the pathogen, such as proteins or peptides, rather than the whole infectious agent. These vaccines typically consist of single antigens or mixtures of antigens that are selected based on their ability to induce an immune response against the target pathogen.
One of the primary advantages of subunit vaccines is their safety profile. Because they contain only specific components of the pathogen, subunit vaccines cannot replicate or cause disease in vaccinated individuals. This makes them particularly suitable for individuals with weakened immune systems or those who may be at higher risk of adverse reactions to live or attenuated vaccines.
However, subunit vaccines are often less effective at inducing immune responses compared to whole agent vaccines. Because they do not contain the entire pathogen, subunit vaccines may elicit weaker or more limited immune responses, particularly cellular immune responses, which are critical for long-term protection against certain pathogens.
Additionally, the production of subunit vaccines can be more complex and costly compared to whole agent vaccines. Purifying specific antigens or proteins from the pathogen requires sophisticated manufacturing processes, which can drive up production costs and limit vaccine accessibility, especially in resource-limited settings.
Another limitation of subunit vaccines is their tendency to require booster doses to maintain immunity over time. Because they may induce weaker or shorter-lived immune responses compared to whole agent vaccines, additional doses are often needed to reinforce and sustain immune memory, ensuring long-lasting protection against the target pathogen.
7.10.6 Adjuvants

Adjuvants play a crucial role in enhancing the immune response to vaccines by targeting pattern recognition receptors (PRRs) on the surface and within the cytosol of immune cells. PRRs are specialized receptors that detect conserved molecular patterns found on pathogens, known as pathogen-associated molecular patterns (PAMPs). By engaging PRRs, adjuvants stimulate innate immune responses and promote the activation of antigen-presenting cells, particularly dendritic cells.
Dendritic cells are key players in the immune system, responsible for capturing, processing, and presenting antigens to T cells, initiating adaptive immune responses. Adjuvants enhance the activation and maturation of dendritic cells, leading to increased expression of co-stimulatory molecules and cytokines. This enhanced activation primes dendritic cells to efficiently present vaccine antigens to T cells, leading to robust and long-lasting immune responses.
Furthermore, adjuvants can help overcome limitations associated with certain vaccines, such as weak immunogenicity or the need for booster doses. By promoting the activation of innate immune pathways, adjuvants can amplify the immune response to vaccine antigens, allowing for the induction of stronger and more durable immunity with lower antigen doses.
7.10.7 mRNA Release

Endosomal escape refers to the process by which mRNA molecules, particularly those encapsulated within lipid nanoparticles (LNPs) used in mRNA vaccines, are released from endosomes into the cytoplasm of host cells. This step is crucial for the successful translation of mRNA into protein and subsequent induction of an immune response.
After the mRNA-LNP complex enters the host cell via endocytosis, it becomes enclosed within endosomes, membrane-bound compartments within the cell. However, for mRNA translation to occur, the mRNA must escape from the endosomes and gain access to the cytoplasm, where the ribosomes, the cellular machinery responsible for protein synthesis, are located.
Several mechanisms have been proposed to facilitate endosomal escape of mRNA-LNP complexes. One proposed mechanism involves the fusion of the endosomal membrane with the lipid membrane of the LNPs, leading to the release of the mRNA into the cytoplasm. Another mechanism involves the disruption of the endosomal membrane by the cationic lipids or other components of the LNPs, allowing the mRNA to escape into the cytoplasm.
Once released into the cytoplasm, the mRNA can be translated by ribosomes to produce the encoded protein, typically the antigen of interest in the case of mRNA vaccines. This antigen is then presented on the surface of the host cell, triggering an immune response and the production of antibodies or activation of T cells against the target antigen.