8 FDA Approval and Post-Market Surveillance
This lecture focuses on the following contents:
- Post-market surveillance (i.e., phase IV)
- Pricing
- Life cycle of drugs and biosimilars
The above figure lists some major drug regulatory agencies in various nations (including Singapore). Also recall that it takes about 12 years for a drug to go from its discovery to market authorization.
8.1 Objectives of Postmarketing Surveillance Trials

The above figure lists some objectives in of postmarket surveillance.
8.1.1 Sales
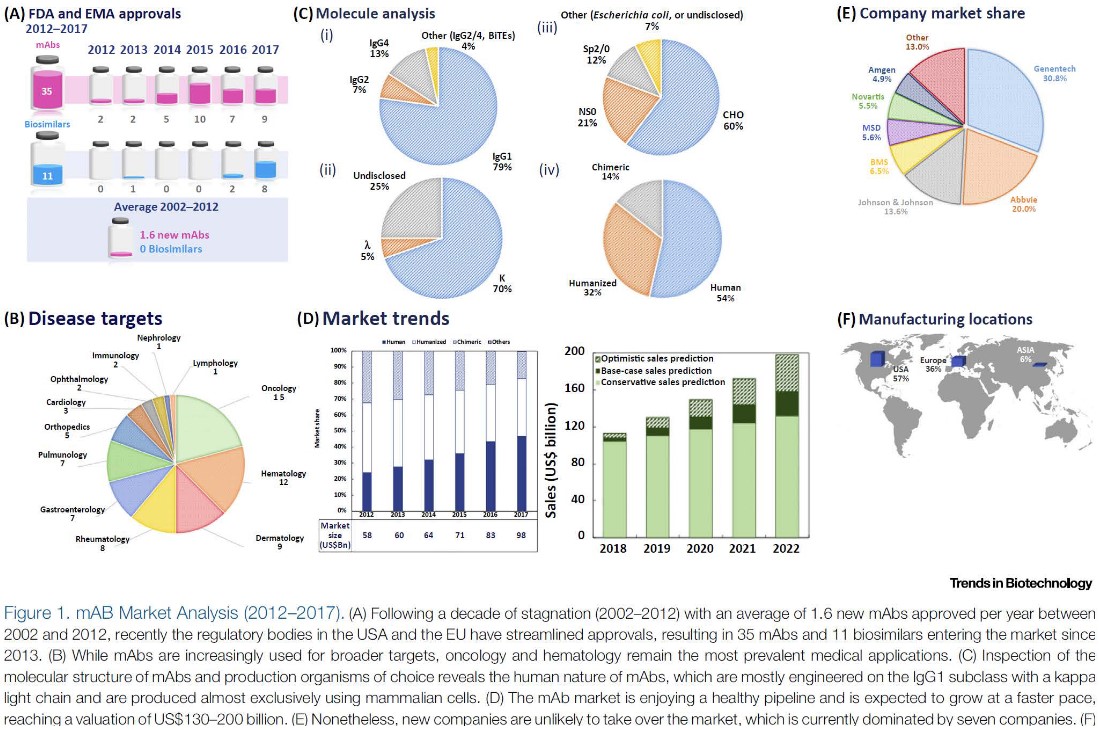
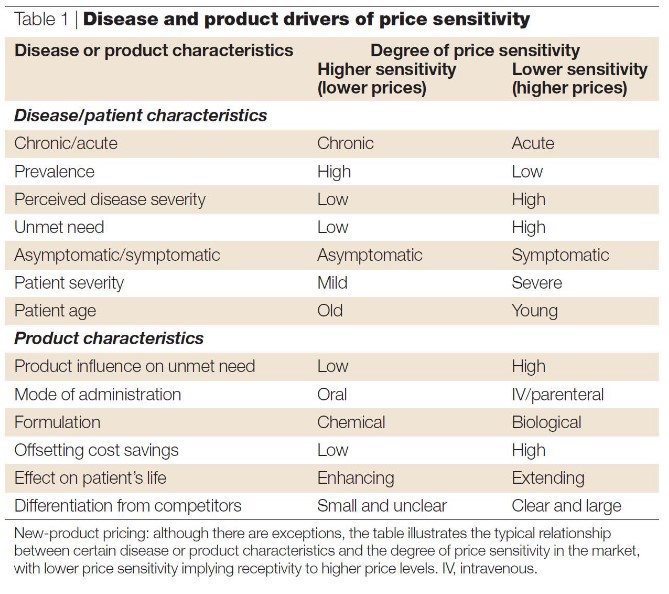
Prices for monoclonal antibodies are also influenced by numerous factors:
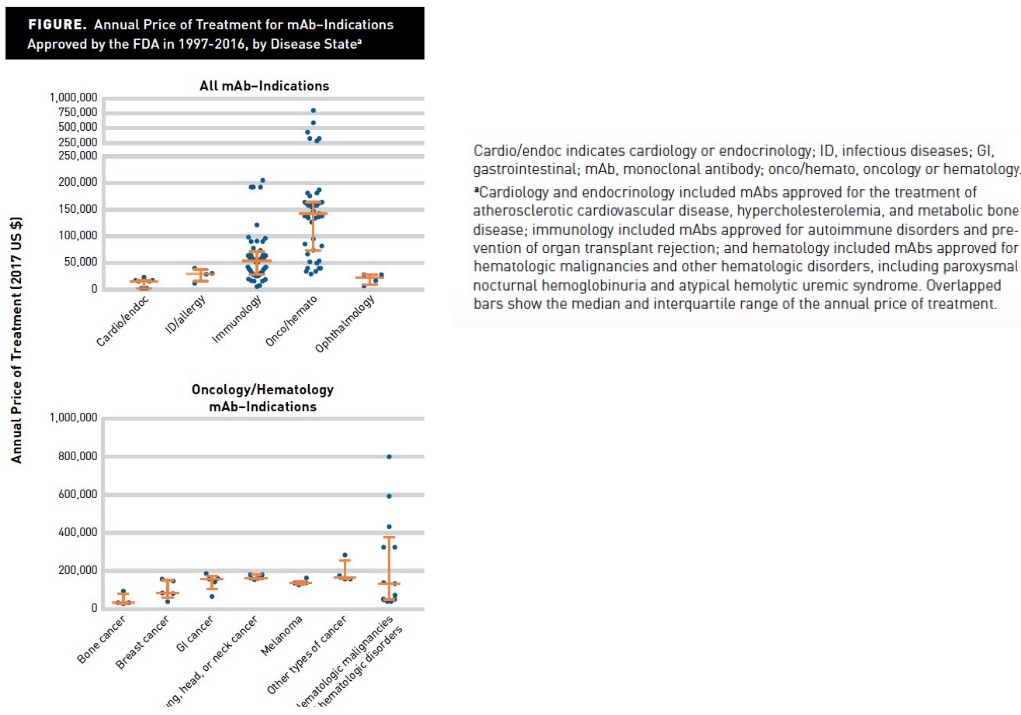
The above figure shows average pricing rates for monoclonal antibodies depending on the kind of treatment that the mAB in question is used for.
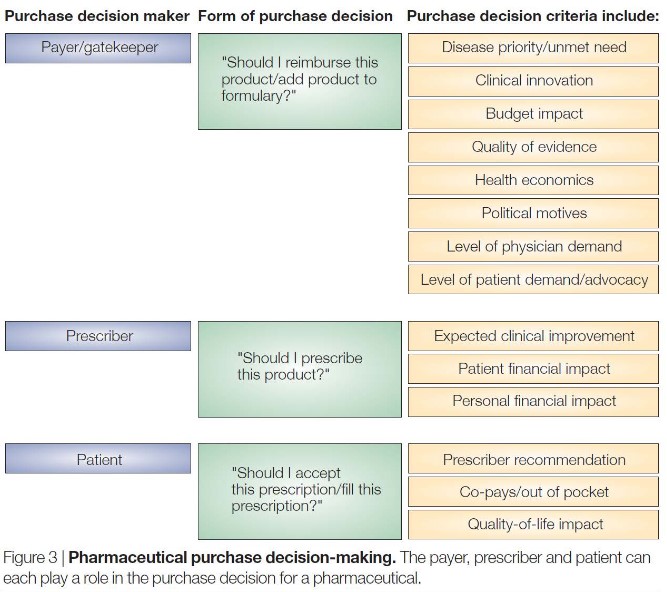
There are also several layers that influence the sale of a monoclonal antibody.
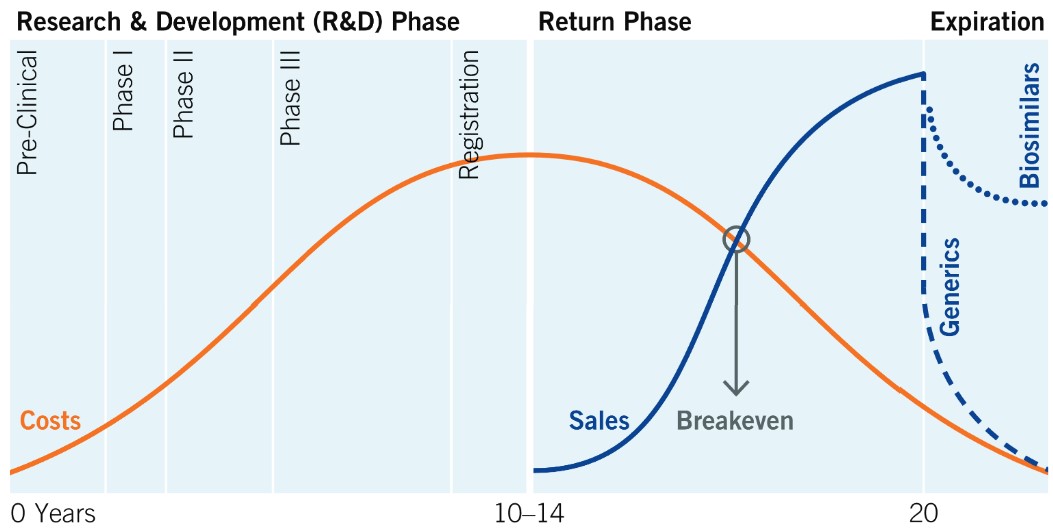
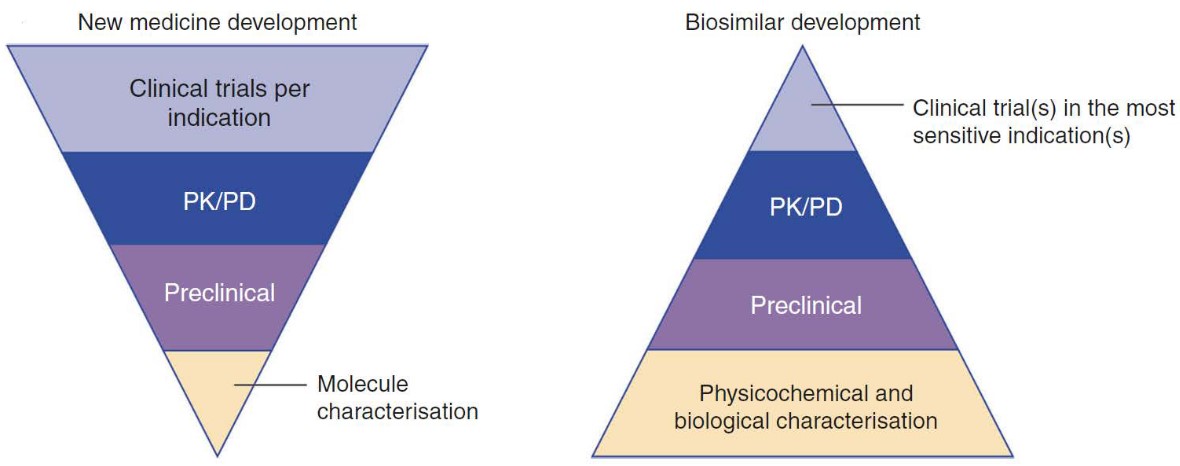
8.2 Lifecycle of Drugs