
7 Clinical Trials for Antibody Therapies
According to the US’ National Institute of Health (i.e., NIH), the main ethical requirements for any study are:
- Social value
- Scientific validity
- Fair subject selection
- Consent
- Favorable risk to benefit ratio
- Independent review
- Respect for human subjects
A thorough review will also be performed by the major regulatory agencies in the above figure (depending on where one is).
7.1 Phase I Studies
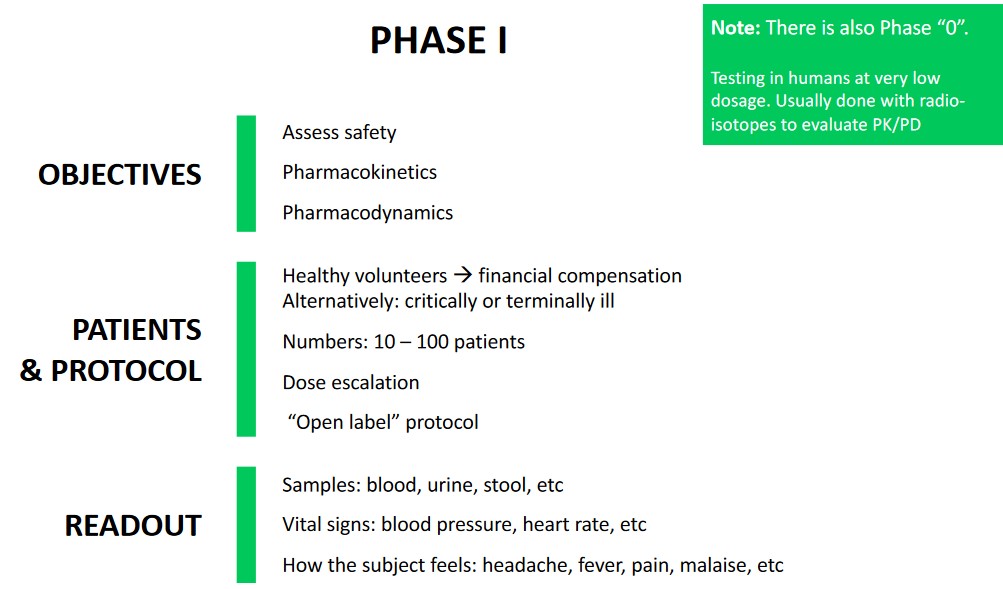
The above figure lists some important ideas to note as they pertain to phase I studies.

The above figure lists five different grades of adverse effects (i.e., AEs).
7.1.1 Examples

Blinatumomab is an antibody that binds to CD3 and CD19 proteins.
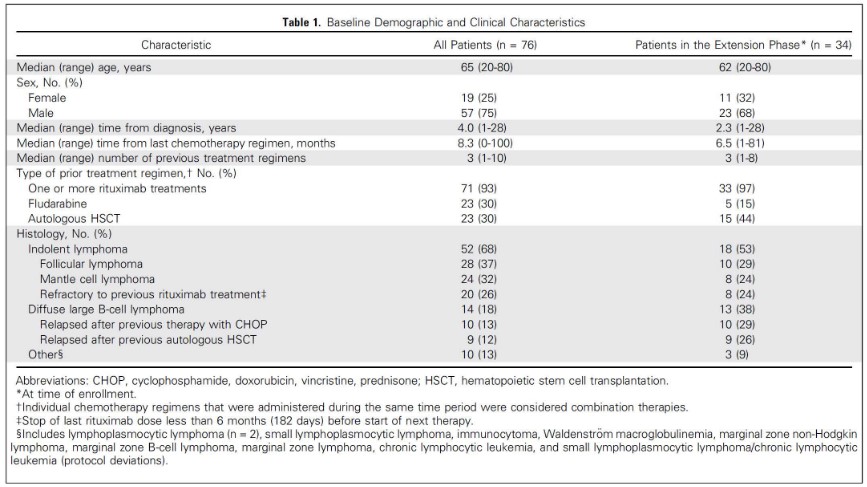
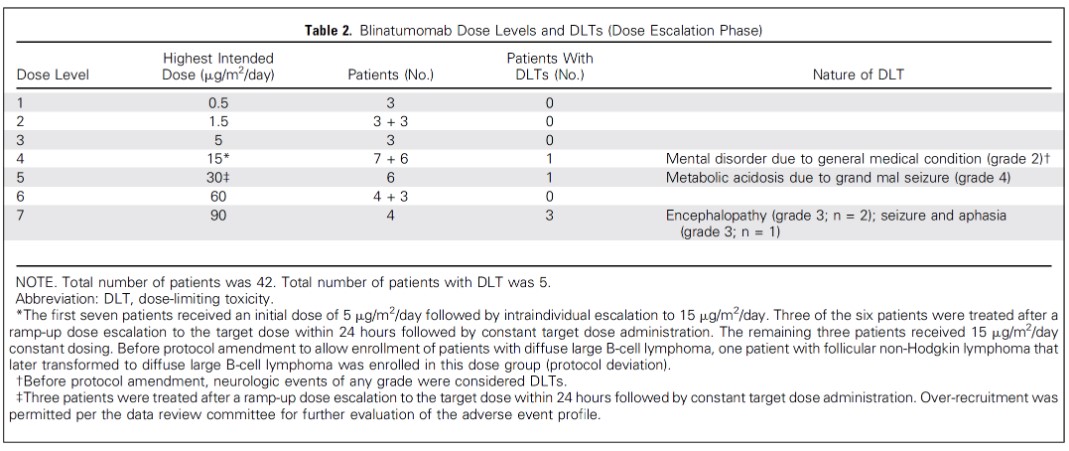

The above figure lists some adverse effects as they pertain to Blinatumomab.
7.2 Phase II Studies



The above graphics list some terms used in phase II studies and the overall objectives of a phase II study.
7.2.1 Examples of Safety and Study Techniques in Phase II
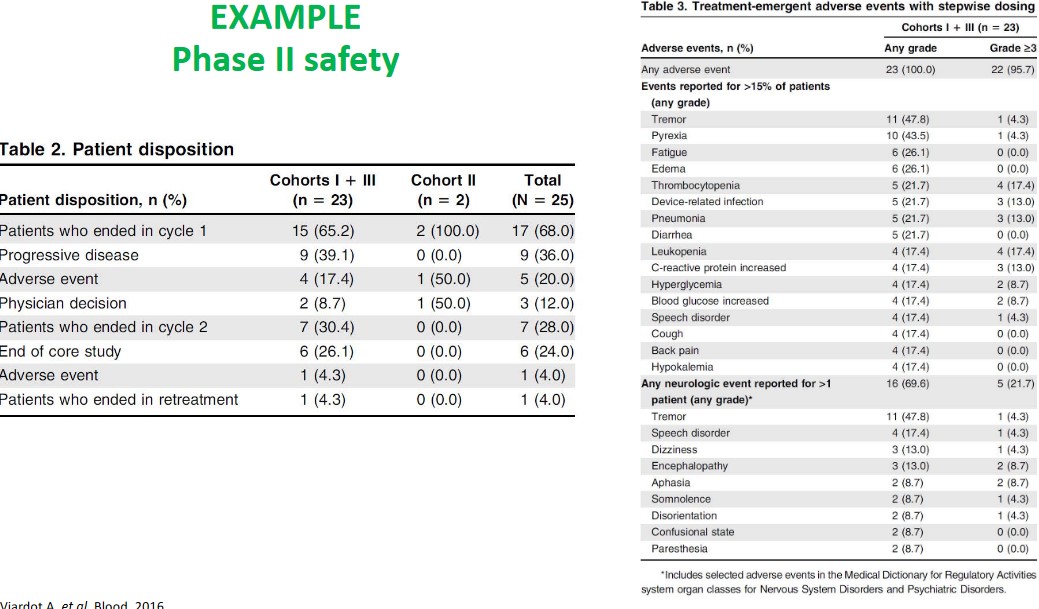
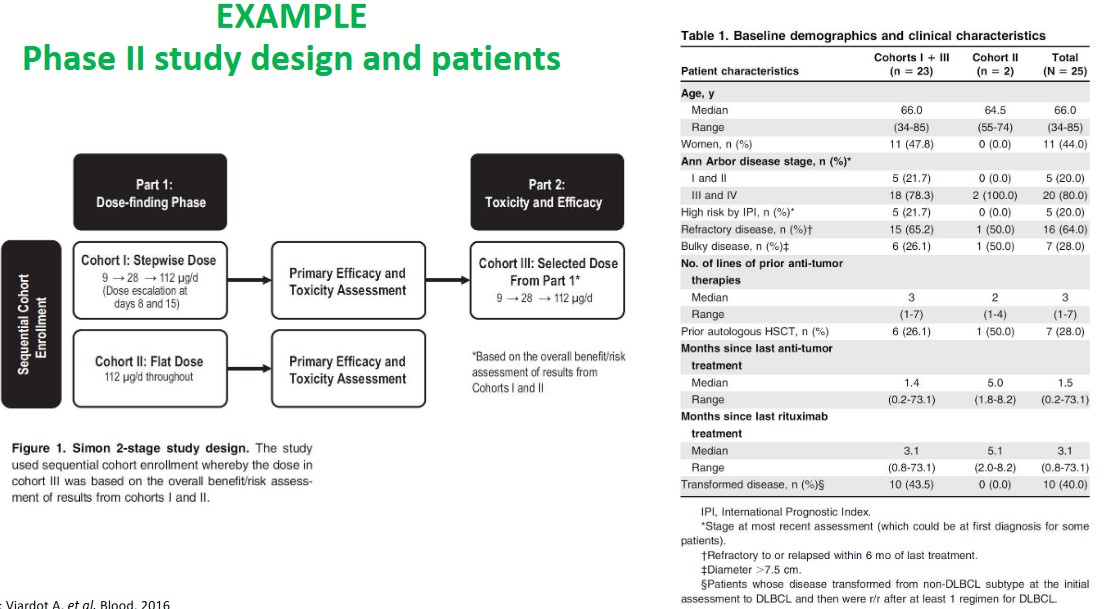
The abvoe graphics show some design and safety considerations when it comes to study design in phase II studies.
7.3 Phase II Studies
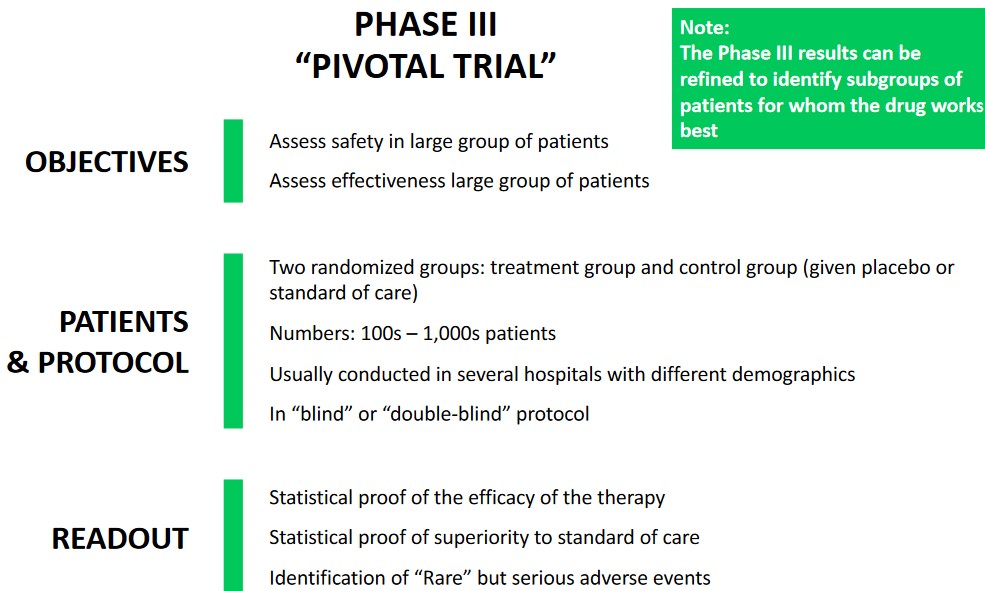
7.3.1 Hazard Ratios

A hazard ratio (i.e., HR) is the proportion of those in the treatment group who have an AE to those in the control who have an AE.
The above graphic also illustrates what different hazard ratios mean.